Telomeres and Aging
advertisement

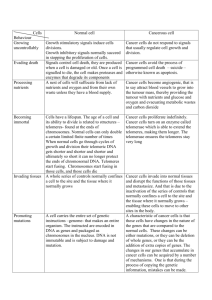
Telomeres and Aging: Is there a connection? What are telomeres? Telomeres are… • Repetitive DNA sequences at the ends of all human chromosomes • They contain thousands of repeats of the sixnucleotide sequence, TTAGGG • In humans there are 46 chromosomes and thus 92 telomeres (one at each end) What do telomeres do? They protect the chromosomes. They separate one chromosome from another in the DNA sequence Without telomeres, the ends of the chromosomes would be "repaired", leading to chromosome fusion and massive genomic instability. Telomere function, cont’. Telomeres are also thought to be the "clock" that regulates how many times an individual cell can divide. Telomeric sequences shorten each time the DNA replicates. Think of it like this…. Telomeres effectively "cap" the end of a chromosome in a manner similar to the way the plastic on the ends of our shoelaces "caps" and protects the shoelaces from unraveling. (Geron corporation) How are telomeres linked to aging? Once the telomere shrinks to a certain level, the cell can no longer divide. Its metabolism slows down, it ages, and dies. Telomeres & Aging Healthy human cells are mortal because they can divide only a finite number of times, growing older each time they divide. Thus cells in an elderly person are much older than cells in an infant. Telomeres & Aging It has been proposed that telomere shortening may be a molecular clock mechanism that counts the number of times a cell has divided and when telomeres are short, cellular senescence (growth arrest) occurs. Telomeres & Aging It is believed that shortened telomeres in mitotic (dividing) cells may be responsible for some of the changes we associate with normal aging. Think of it like this… Geron Corporation likens the telomere and aging condition to this: • For the cell, having a long telomere can be compared to having a full tank of gas in your automobile; having a short telomere is like running on empty. Each time a cell divides, its telomeres become a little shorter until the cells simply can no longer divide (e.g., it runs out of fuel). Telomeres & Aging “After a certain number of cell divisions, the telomeres would be so short as to somehow prevent the cell from further proliferation--putting it in a state called senescence. In other words, he proposed that telomere length offered a clock for telling a cell's longevity.” - Scientific American What next? So, scientists have determined that there is a direct connection between telomere length and aging. What was their next step? What Next? Dr. Jerry Shay and his colleagues (The University of Texas Southwestern Medical Center at Dallas ) found that cellular aging can be bypassed or put on hold by the introduction of the catalytic component of telomerase (i.e., the fuel added to the gas tank to keep the car running)! What is telomerase, anyway? Telomerase (TEE-LÓM-ER-ACE) is a ribonucleoprotein enzyme complex (a cellular reverse transcriptase) that has been referred to as a cellular immortalizing enzyme. It stabilizes telomere length by adding hexameric (TTAGGG) repeats onto the telomeric ends of the chromosomes, thus compensating for the erosion of telomeres that occurs in its absence. So how does this all link together? In the laboratory, cells in tissue culture with introduced telomerase have extended the length of their telomeres. They have already divided for 250 generations past the time they normally would stop dividing, and are continuing to divide normally, giving rise to normal cells with the normal number of chromosomes. How Does Telomerase Work? Telomerase works by adding back telomeric DNA to the ends of chromosomes, thus compensating for the loss of telomeres that normally occurs as cells divide. Most normal cells do not have this enzyme and thus they lose telomeres with each division. How Does Telomerase Work? In humans, telomerase is active in germ cells, in vitro immortalized cells, the vast majority of cancer cells and, possibly, in some stem cells. High telomerase activity exists in germ cells, stem cells, epidermal skin cells, follicular hair cells, and cancer cells. How Does Telomerase Work? Research also shows that the counter that controls the wasting away of the telomere can be "turned on" and "turned off". The control button appears to be an enzyme called telomerase which can rejuvenate the telomere and allow the cell to divide endlessly. Most cells of the body contain telomerase but it is in the "off" position so that the cell is mortal and eventually dies. How Does Telomerase Work? Some cells are immortal because their telomerase is switched on Examples of immortal cells: blood cells and cancer cells Cancer cells do not age because they produce telomerase, which keeps the telomere intact. Visual Example The next slide shows cells stained to visualize the presence of telomerase. The bottom dish was treated to produce active telomerase and is still dividing The top dish of normal cells of the same age has stopped dividing Telomerase and Cancer There is experimental evidence from hundreds of independent laboratories that telomerase activity is present in almost all human tumors but not in tissues adjacent to the tumors. Telomerase and Cancer Thus, clinical telomerase research is currently focused on the development of methods for the accurate diagnosis of cancer and on novel anti-telomerase cancer therapeutics Experimentation Many experiments have shown that there is a direct relationship between telomeres and aging, and that telomerase has the ability to prolong life and cell division. Genetic Link The telomerase control gene has been mapped to 3p21 (chromosome 3, the p (short) arm, locus 21) Although the gene for telomerase is present in all cells, hTRT is present only in immortal cells, where it serves to fuse the repeating sequences of DNA to the chromosomes, thereby lengthening the telomeres. Genetic Link Proof that introduction of the hTRT gene into mortal cells would cause them to produce active telomerase was offered in the December 1, 1997, issue of Nature Genetics by Genron researchers This info brought to you by: Elizabeth Jordan Sources (1 of 4) Reveal P J, Henkels K M, and Truchi J J. 1997 "Synthesis of the Mammalian Telomere Lagging Strand in Vitro." Journal Of BioChemistry Vol. 272:No. 18 pp1167811681 Scientific American “Turning Back the Strands of Time” Sources (2 of 4) McElligott R, and Wellinger R J. 1997 "The Terminal DNA Structure of Mammalian Chromosomes." EMBO Journal Vol. 16:no. 12 pp 3705-3714. (www.emboj.org/) “Mapping and Cloning of a Human Telomerase Repressor Gene on Chromosome 3" CS Cooper et. all Sources (3 of 4) Geron Symposium No. 3 “Telomerase and Telomere Dynamics in Cancer and Aging” (www.geron.com/) “Mouse model demonstrates role of telomeres and telomerase in aging, cancer and lifespan” (http://www.arclab.org/ March 4, 1999 ) Sources (4 of 4) “Shay/Wright Laboratory” The University of Texas Southwestern Medical Center at Dallas. (www.swmed.edu/) “Aging and Cancer: Are Telomeres and Telomerase the Connection?” (http://www.accessexcellence.org/LC/ST/st 10bg.html)