Week of: September 29 – October 3, 2014 Unit 2: Chemical Bonding
advertisement
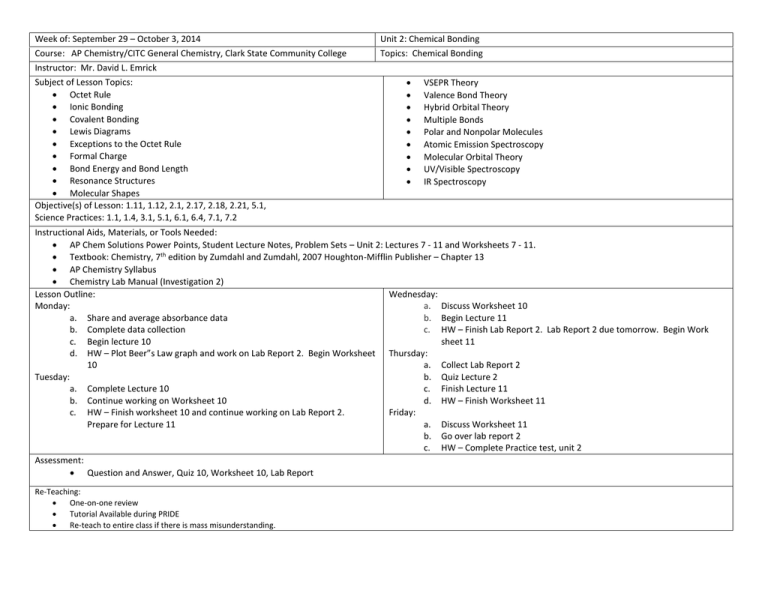
Week of: September 29 – October 3, 2014 Course: AP Chemistry/CITC General Chemistry, Clark State Community College Instructor: Mr. David L. Emrick Subject of Lesson Topics: Octet Rule Ionic Bonding Covalent Bonding Lewis Diagrams Exceptions to the Octet Rule Formal Charge Bond Energy and Bond Length Resonance Structures Molecular Shapes Objective(s) of Lesson: 1.11, 1.12, 2.1, 2.17, 2.18, 2.21, 5.1, Science Practices: 1.1, 1.4, 3.1, 5.1, 6.1, 6.4, 7.1, 7.2 Unit 2: Chemical Bonding Topics: Chemical Bonding VSEPR Theory Valence Bond Theory Hybrid Orbital Theory Multiple Bonds Polar and Nonpolar Molecules Atomic Emission Spectroscopy Molecular Orbital Theory UV/Visible Spectroscopy IR Spectroscopy Instructional Aids, Materials, or Tools Needed: AP Chem Solutions Power Points, Student Lecture Notes, Problem Sets – Unit 2: Lectures 7 - 11 and Worksheets 7 - 11. Textbook: Chemistry, 7th edition by Zumdahl and Zumdahl, 2007 Houghton-Mifflin Publisher – Chapter 13 AP Chemistry Syllabus Chemistry Lab Manual (Investigation 2) Lesson Outline: Wednesday: Monday: a. Discuss Worksheet 10 a. Share and average absorbance data b. Begin Lecture 11 b. Complete data collection c. HW – Finish Lab Report 2. Lab Report 2 due tomorrow. Begin Work c. Begin lecture 10 sheet 11 d. HW – Plot Beer”s Law graph and work on Lab Report 2. Begin Worksheet Thursday: 10 a. Collect Lab Report 2 Tuesday: b. Quiz Lecture 2 a. Complete Lecture 10 c. Finish Lecture 11 b. Continue working on Worksheet 10 d. HW – Finish Worksheet 11 c. HW – Finish worksheet 10 and continue working on Lab Report 2. Friday: Prepare for Lecture 11 a. Discuss Worksheet 11 b. Go over lab report 2 c. HW – Complete Practice test, unit 2 Assessment: Question and Answer, Quiz 10, Worksheet 10, Lab Report Re-Teaching: One-on-one review Tutorial Available during PRIDE Re-teach to entire class if there is mass misunderstanding.