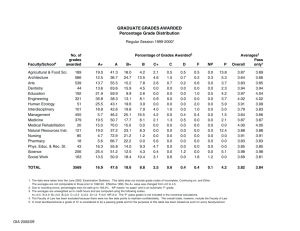
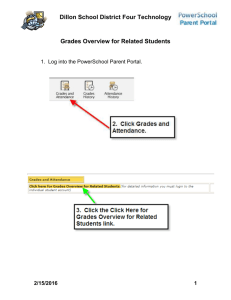
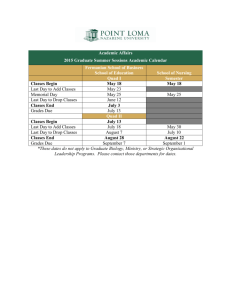
Science Dept Hand book 2012 - 13 - Assessing
advertisement

Science Department Handbook 2012-13 Contents Overview Page 2 Science Curriculum Statement Page 4 Personnel Page 5 Staffing and teaching allocations Professional Development Appraisal Absence Resources Page 7 Textbooks Photocopying Practical Equipment Issue to students Issue to Teachers Teacher resources Computers Assessment and Reporting Page 10 Types of assessment Organisation of assessments IGCSE coursework IB coursework The Group 4 Project Recording of assessment results Reporting to Parents Presentation of written work Policies Health and Safety Page 13 Homework Marking of work Presentation of work Classroom Routines and Environment Rewards and Sanctions Contact books Appendices HEALTH & SAFETY HANDBOOK Page 16 Student Study Guide TO BE POSTED ON MOODLE Meetings format Science exam results report 2008-2011 Practical equipment list Page 29 Page 46 Page 48 1 Overview The Sultan’s School is highly regarded in the community. It is widely recognized as a school of standing by the community and also throughout the country. It aims to be the best International school both in Oman and in the Middle East. It is a fee paying school and students sit an entrance examination in order to procure a place in the school. In the main, parents are very keen to ensure that their children achieve the highest grades. School Mission Statement The Sultan’s School is a co-educational school offering bilingual Arabic-English education from early childhood to pre-university. The school seeks to provide a broad and balanced education of the highest quality, which reflects and strengthens the Omani and Islamic culture while encouraging an international perspective and developing critical, creative thinking in its students. Statement of Philosophy: The Sultan’s School exists to help young men and women acquire those ethical and intellectual qualities that are necessary for leadership in the rapidly changing world of Oman. While the focus is upon developing these qualities in Omani citizens, qualified students from other countries are welcome. The educational programme will endeavour to produce versatile leaders who are international in their perspective. The school will be preparatory in nature since it is assumed that most graduates will attend a university. The total school programme will encourage each student to become a self-directed individual who assumes responsibility for his or her actions. Within the instructional programme, there must be a delicate balance between maintaining standards and offering individual programmes to meet the varying abilities, learning style and previous achievement of each student. Every attempt will be made to identify ability and nourish its development. Science Staff There are eleven fulltime Science teachers and five Laboratory Technicians. At present, there are teachers from England, Canada, Wales and Zimbabwe and Zambia. The teachers bring a varied set of experiences to the Science Department. They have experience of having taught in many different countries and consequently students from a variety of cultures. They have taught a variety of courses. The teachers are experienced, friendly and supportive of each other and the department in general. Together we form a strong team. Students The Science Department works with two different student groups: the Sultan's School students and the Advanced Learning Centre students; (A-level Physics and Chemistry). Although these courses cease to run after 2012-13. The majority of the students in the Secondary section of The Sultan's School have progressed from the Elementary section. Their level of English is generally good and they are taught Science in English within a bilingual programme. There are approximately 50 students in Years 7 to 13 who board at the school. These students are known as the scholars and have been chosen on the basis of their achievement in their local 2 Government schools and are sponsored principally by His Majesty The Sultan. They are taught by a specialist ESOL teacher for English. The Advanced Learning Centre (ALC) students have successfully completed the government secondary school curriculum delivered in Arabic. They are selected by Shell and the local oil company, Petroleum Development Oman (PDO), on the basis of ability and aptitude. There is a range of English language ability amongst these students but the ALC has specialist 'English for Academic Purposes’ teachers who work in tandem with the Maths and Science teachers. Parents The parents of the students at The Sultan’s School are extremely interested in and concerned about their children’s education. Turnout at parent interviews is reasonable. Parents are keen to assist in any way they can. They view education as of prime importance and wish to be kept informed of student progress. Facilities in the Science Department There are currently nine Science laboratories: Four sited in the main school complex and five in a building known as the ALC Centre (80 metres from the school site). The school site laboratories comprise two Biology labs and two General Science labs. At the ALC there are two Physics labs, two Chemistry labs and a General Science Lab. Both sites have excellent preparatory rooms manned by our superb technical team. The labs are very well resourced and maintained, air conditioned and contain white boards, storage cupboards or shelves, a ceiling mounted projector + laptop computer. Six of the nine labs are equipped with computers with internet and intranet access for student use. There is a 'Science Staffroom' at the School site currently equipped with 9 study areas and tea & coffee making facilities. Science Curriculum The curriculum for Years7, 8 and 9 is based on the English National Curriculum and delivered through the 'Science Works' scheme. At the IGCSE options evening towards the end of Year 9, the students are given the opportunity to choose one, two or three Sciences at IGCSE based on recommendations from their Science teachers, these recommendations are largely driven by the students' performances over the three year KS3 course. Years 10 and 11 Biology, Chemistry and Physics follow the IGCSE Cambridge Examining Board specification, sitting the examinations at the end of Year 11. Year 12 and 13 students work towards the International Baccalaureate diploma and certificate. Students choose one or two Science courses at IB. The ALC students study at The Sultan’s School for two years. During that time they are examined in the units of the EDXCEL A Level in Physics and Chemistry. 3 Science Curriculum Statement Science is a dynamic, forward looking, collaborative human endeavour that provides a distinctive way of thinking about and explaining the events and phenomena in the world. Science education at the Sultan's School aims to foster students' curiosity, imagination and wonder. The study of Science has lead to an evolving body of knowledge which has been, and continues to be, built up over time and revised as new evidence comes to light through the practice of Science. It provides explanations for a variety of phenomena that enable us to make sense of the biological, physical and technological aspects of our world, predict future events, as well as develop and implement technologies that improve the quality of life. Science education helps students reach a scientific understanding of their world. It provides them with the skills and cognitive abilities to access the ever expanding body of knowledge in order to better understand themselves, local and global sustainability and other issues. Science education involves students using a range of technologies, including ICT to explore their world. At The Sultan's School we encourage the students to approach Science as a process of inquiry which involves questioning, predicting, hypothesizing, investigating and gathering evidence, organizing data to elicit patterns, testing and refining ideas, developing explanations for natural phenomena and communicating these to others. As students acquire the skills of working scientifically, they develop an understanding of evidence and the nature and practice of Science. Working both individually and in teams, students are engaged in critical and creative thinking, to solve problems and clarify ideas. 4 Personnel Staff and teaching Allocations 2012-13 Physics Alan Jones Head of Physics Mark Power Ayub Qureshi Chemistry Trevor Blackman. Head of Science Faculty Ian Bevis Head of Chemistry Olivia Prince Kathryn Norton Coordinator of Key Stage 3 Science Biology and Environmental Systems Emmajane Trickett Head of Biology John Mutoko Sarah Razzaq Key Stage 3 The following teachers from the above list are currently teaching Years7, 8 & 9 Teams: Year 7: Olivia. Kathryn, Sarah Year 8: Olivia, Kathryn, Emmajane, John Year 9: Trevor, Ian, Kathryn, John, Sarah, Alan, Mark, Ayub Teams responsible for production and maintaining relevant resources under the guidance of Trevor. 5 Professional development Professional development takes the form of: external courses, whole school inset, Department meetings, teaching team discussions, appraisal. IB Training All members of the department should update their knowledge of the IB curriculum and procedures every two years. When possible and appropriate the school will offer the opportunity for staff to attend IB approved courses throughout the year. Whole school Whole school inset time will provide an opportunity for staff to: receive information from other departments and sections within the school discuss and share ideas on how to improve the school discuss how to meet the school aims Department meetings (See appendices for formats) Whole Science Department meetings are convened when necessary : Prior to / after Middle Managers meetings At key dates during the school calendar: prior to key assessments, parent's evenings, open days. Meetings of the teaching teams in Chemistry, Physics, Biology and Key Stage 3 are held more frequently: usually every week to review progress and plan future policy. Appraisal Each year, teachers are observed by the Head of Secondary and the Head of Department. The observation and evaluation forms provide information for the principal who meets with each teacher after the information has been gathered. After the appraisal process is completed, each staff member is required to decide on at least two objectives relating to their teaching and contribution to the Department. Absence The ‘Academic Coordinator’ is responsible for providing cover. If a teacher is going to be absent, He must be phoned before 7 am the morning of the absence, on 96078831. Cover for classes must be given to the Head of Department before school begins. Any teacher who knows in advance that they will be absent, must complete a form and pass it to The Academic Coordinator as soon as possible. 6 Resources Textbooks Textbooks in current use Key Stage 3 Year 7 Science Works 1 (Oxford) Year 8 Science Works 1 & 2 Year 9 Science Works 2 & 3 An extensive range of topic based course booklets/ study guides have been developed and are used far more frequently than the textbooks. IGCSE Physics Chemistry Biology Cambridge IGCSE (Collins) Cambridge IGCSE Chemistry Cambridge IGCSE Biology (Collins) (Collins) IB Physics Chemistry Biology Physics Course Companion Chemistry Course Companion Biology Course Companion (Oxford) (Oxford) (Oxford) A Level Physics Edexcel Physics (Pearson/Heinemann) Chemistry Edexcel Chemistry (Pearson/Heinemann) An extensive range of topic based course booklets/study guides have been produced by departments and are used in conjunction with the textbooks. Text book orders and allocation The HoD places an order for textbooks with Yahya (the Director of Finance) prior to January of each year. All textbooks need to be stamped and numbered. New textbooks will be numbered from 1 and include the year of purchase i.e. 34/11. There is a space on the stamp to record the condition of the text. Books are expected to last for three years and are allocated the following conditions to encourage this. Condition 1 is for new books, condition 2 is for good condition books, condition 3 is for fair condition books. 7 Textbooks are issued to Years 7 to 10 and 12 at the beginning of the school year. Teachers and students complete a book issue form and send this form to the Senior Laboratory Technician.. Students should complete the stamp in the textbook by writing their name, date of issue and condition of the book as determined by the teacher. Spare books will be kept in the Science storeroom. Year 10 and 12 students are issued with textbooks. These textbooks are their own property to write notes in and use as they wish. Students sign for a text and the list is given to Yahya who charges the students? Textbooks for ALC students are kept in the ALC store room. Textbooks are issued to the ALC students at the commencement of each year. These textbooks are their own property to write notes in and use as they wish. Students sign for a text on a book issue form from the ALC staffroom. At the end of the school year or when a student leaves, the technicians collect texts and check off their return on the book list. A list of students who fail to return a text or return a text in poor condition should be given to Yahya . Texts lost or damaged during the year must be paid for. Students should sign for a new text and Yahya should be informed so that he can charge the student. Photocopying The photocopier in the ALC administration block should be used for ALC material and for small numbers of other material. In the main school block, the photocopier in the main staffroom or in Nicholas’ office next to the main staffroom should be used if teachers want to copy material. For booklets, materials prepared in advance, exams and tests, the photocopy request form should be completed, attached to the master and placed in Nicholas’ pigeonhole in the main staffroom. Nicholas will place the completed work in pigeon holes or on the bench in his office. The Science code for the ALC photocopier is 69790, the code for the photocopier outside Nicholas’ office is 5050, the code for the staffroom photocopier is ……….. Issue to students At the beginning of the school year, students are given one large file, lined and graph paper. After that it is the students’ responsibility to provide all equipment. Stationary is available from the school store. The order for students files, paper etc is given to Salam; the storekeeper prior to the start of the year. Issue to Teachers At the beginning of the school year, staff are issued with basic stationary. To order stationery throughout the year complete a stationery request form, have it signed by the HoD and place it in Salam’s pigeon hole. He will put the requested items into pigeonholes. Teachers’ planners or mark books may be obtained from Nicholas (Reprographics Manager). Keys for the labs are issued by Ali. 8 Practical equipment A list of available equipment can be viewed in the appendix. Storage of paper resources There are numerous filing cabinets in the Science and ALC staffrooms. The Physics, Biology, Chemistry and KS3 Departments have prioritised the use of these cabinets as they see fit. Computers There are PC's with network and internet access and laser printers available for general staff use in the School and ALC workrooms. All teachers are issued with a code which enables them to access the internet and the school network. To access the Science folder follow the following path. ENTIRE NETWORK/……….. / SCHOOLS/SECONDARY/ SCIENCE. Each teacher has a personal folder can only be accessed only by the relevant teacher. To access personal folders the following path needs to be followed Every room has a computer, and a projector. Colleagues can be contacted through the intranet. Help with the computers can be obtained from support staff in the 'Help room' There are two computer labs in the ALC block and one in the Green Block that can be booked for student use when they are not being used by the ICT department. 9 Assessment and Reporting Types of Assessment There are numerous types of Science assessments. These include: End of Year Examinations, Unit Tests, Quizzes, Investigations, Research Projects, Experimental Work, Presentations, Homework. The whole school assessment and marking policy is under review, but in the interim the following is expected: Years 7, 8 and 9 Years 7,8 and 9 are given a test on the completion of each topic and a common examination at the end of the year. The general timing of tests varies according to topic length, the tests satisfy the APPs in the English National Curriculum and are appropriately ‘levelled’. The timings of examinations are written into the school calendar. In 2012-13 it is expected that each Year Group will complete one major Investigation / Piece of project work that will be appropriately assessed, displayed and presented to their peers. The purpose of these assessments is both diagnostic and informative. As they mark their students work, teachers need to identify group areas of weaknesses as a basis for further work less able students who need additional help, students whose performance has slipped students who may need extension This information should be used to help students to achieve to their potential and to make sure that gaps in learning are filled by further work and explanation. Students need to be encouraged to look critically at their results, to identify areas of weakness and then to take responsibility for learning the work they scored badly on, by seeking help and practicing those skills and concepts. Years 10 to 13 Quizzes/Tests are set at the discretion of the separate Departments. Years 10 and 12 have end of year examinations, Years 11 and 13 have a mock IGCSE/IB examination in February and then their final external examinations in May/June. ALC Tests will be set as and when appropriate: Usually Topic based. Currently the Year 1 students sit Physics 1,2,3 and Chemistry 1,2 at the end of their first year. The Year 2 students sit Physics 4,5,6 and Chemistry 3,4,5,6 in December / June at the Department's disgression. IGCSE Coursework (see appendix) This practical component of the IGCSE course contributes 20% of the total marks available. Currently the Chemistry, Biology and Physics Departments follow the 'Alternative to Practical' scheme, with students sitting the Alternative to Practical examination. 10 IB Coursework (see appendix) This component comprises 24% of the total marks available. A minimum of 60 hours laboratory work (Higher Level) and 40 hours (Standard Level) is expected to be completed over the duration of the course. It is the Department's responsibility to ensure that a suitable Practical Scheme of Work is in place and that the criteria for assessment are satisfied. The Group 4 Project Every IB Science student is expected to take part in an interdisciplinary 'Group 4 Project'. Currently this is facilitated at the Sultan's School by a series of investigations being carried out over two full days by the IB1/Year 12 students at the very end of their first IB year. Recording Assessment results Assessment results for each Year/Option group are recorded on Department's spreadsheets. It is the responsibility of each teacher and the Head of Department to ensure that the records are up to date. The results can be found in the folder names with the appropriate school year. Members of the Leadership team and Heads of Year have access to these records. Reporting to parents Parent Interviews are conducted twice a year. Teachers need to take a printout of results which needs to include the previous year’s result, and a copy of the most recent reports. Parents wish to know when their child is having problems with the material taught. When there is a deterioration in results, parents appreciate being told as students do not always tell their parents. Usually there is a marked improvement in application after parents are informed. It is particularly important that parents of IB and IGCSE students are informed by phone or email if there is a deterioration in performance. Assessment results for ALC students are recorded in the ALC database which is set up by John Watson (Director of the ALC). This is used as a basis for the biannual report on student progress which is sent to the PDO liaison person. As well as teachers writing individual reports, the Head of Department has to write a year group report. All report forms are computer generated. Reports are accessed by clicking on the SSM icon on the desktop. The username is t followed by the teachers three letter initial. Teachers are given a password that can be changed after the initial login. A timetable is issued by senior management setting out the deadlines which must be met. Teachers are expected to write at least three sentences outlining progress achieved, application, some aspect that can be improvement. It is important that reports state the truth but are professional. Teachers are expected to complete their reports to the given deadline and pass them onto a buddy to proof read. After printing, the HoD proof reads the Science reports and return reports for corrections. The reports are then proof read by the Head of Secondary who makes minor changes or returns the report to the writer for correction. All reports require teachers to give a grade for effort and achievement. On the progress reports at the beginning of the school year, teachers are required to write S (satisfactory), D (borderline) or N (not satisfactory) for student achievement and to write brief written comment. On Year 7 to 10 reports teachers are expected to write a grade of A to E. In Years 7,8 and 9 the grade boundaries will be decided after the results for each year group are considered collectively by 11 teachers of that year group. The grades in Year 10 and 11 will very much reflect the previous year’s Grade boundaries for the IGCSE examination. The Years 7, 8 and 9 achievement grades will be considered in the light of future IGCSE grades. IB students are given a grade from 1 to 7 in line with the previous IB examination grade boundaries. Presentation of Written Work At The Sultan’s School we set high expectations for the students and aim to create a consistent approach for them in the setting out of their work. This has to be done from the beginning but in a way which is appropriate for their age and experience. Students should receive every encouragement to take pride in their work and its presentation. Years 7 and 8 Work should be done in an exercise book 1. The date should be written at the top left-hand side of the page. This should be in the English ordinal form e.g. 12th February. The inclusion of the year is optional. 2. The title should be centrally placed and then a line or two left before beginning writing. The students should be taught the English rules for using capital letters in titles. 3. All headings should be underlined with a ruler. A different colour may be used. 4. A piece of work may be ruled off with a ruler, if appropriate. At the bottom of the page, this is unnecessary. 5. Pupils should not use Tippex, correcting fluid or eraser pens. When an error is made in pen, it should be crossed out with a single line. Brackets are not appropriate. 6. Students should write in pen. Students can use fountain pens: Berol handwriting pens are acceptable. These are available from the School Shop. 7. Where appropriate, corrections should follow each piece of work. 8. When work continues onto a second page, students should normally use the top line of the page. 9. A missed line should indicate where a new paragraph begins, rather than an indentation. 10. Students should be discouraged from dividing a word at the end of a line if they have insufficient space. They should opt for beginning the word on the next line. 11. High standards of handwriting are expected. Years 9,10,11 Work may be done in files if the students prefer or Word Processed and in e-form if appropriate. All e-documents must have the Student’s name on the top right hand corner and follow the rules for written work in a book. Guidelines as above Years 12 and 13 Work may be in files or in e-format. Work may be stored in e-format and only printed when requested. 12 Department Policies Health and Safety Health and Safety is obviously paramount in the Science Department. Please refer to the detailed document: ‘Health and Safety’ in the appendices. Homework Homework provides students with the opportunity to practice and apply the skills that they have learned in class. It is an opportunity to reinforce learned skills, not to attempt new skills. It should be work that students can cope with and complete with reasonable confidence. Homework provides teachers with feedback on how well students have mastered recently taught concepts and skills, and to identify where further teaching is needed. To show the value that the department places on to homework, teachers should check that it has been completed and feedback given on a regular basis. This feedback may be given on an individual or class basis during class. To alleviate the inevitable ‘collaboration’ between students over homework exercises the concept of ‘pop’ quizzes has often been successfully employed: The students are set a small amount of topic work to review for homework, then quizzed briefly for 10-15 minutes at the start of the next lesson. Presentation of Written Work At The Sultan’s School we set high expectations for the students and aim to create a consistent approach for them in the setting out of their work. This has to be done from the beginning but in a way which is appropriate for their age and experience. Students should receive every encouragement to take pride in their work and its presentation. Years 7 and 8 Work should be done in an exercise book 1. The date should be written at the top left-hand side of the page. This should be in the English ordinal form e.g. 12th February. The inclusion of the year is optional. 2. The title should be centrally placed and then a line or two left before beginning writing. The students should be taught the English rules for using capital letters in titles. 3. All headings should be underlined with a ruler. A different colour may be used. 4. A piece of work may be ruled off with a ruler, if appropriate. At the bottom of the page, this is unnecessary. 5. Pupils should not use Tippex, correcting fluid or eraser pens. When an error is made in pen, it should be crossed out with a single line. Brackets are not appropriate. 6. Students should write in pen. Students can use fountain pens: Berol handwriting pens are acceptable. These are available from the School Shop. 7. Where appropriate, corrections should follow each piece of work. 13 8. When work continues onto a second page, students should normally use the top line of the page. 9. A missed line should indicate where a new paragraph begins, rather than an indentation. 10. Students should be discouraged from dividing a word at the end of a line if they have insufficient space. They should opt for beginning the word on the next line. 11. High standards of handwriting are expected. Years 9,10,11 Work may be done in files if the students prefer or Word Processed and in e-form if appropriate. All e-documents must have the Student’s name on the top right hand corner and follow the rules for written work in a book. Guidelines as above Years 12 and 13 Work may be in files or in e-format. Work may be stored in e-format and only printed when requested. Marking Policy At present there is no fixed Departmental Marking Policy, except that all pieces of work generated by students should be corrected / marked appropriately. The style of marking will vary in degree of rigor according to the nature of assignment. However, marking should be: Focused on pupil learning Based on shared learning objectives Periodically and selectively given Positive in tone and accessible by all pupils Supportive of achievement in all its forms Helping pupils to improve their work Promoting learner confidence Including opportunities to develop peer and self assessment skills Informing future planning and thereby support individual ‘target getting’ 14 Classroom routines and environment It is the responsibility of each teacher to ensure that their classroom provides a Science environment. Anyone walking into the classroom should immediately be aware that this is a room where the learning of Science takes place. Displays of student work, current course information and posters, should be used to make the room stimulating and attractive. The relationship with the teacher is key to creating a positive learning environment. The classroom should be a safe and calm place where students feel motivated, know that they are treated with respect and where they treat all others with respect. It should be a place where they can make mistakes without ridicule and learn from their mistakes. It is important that: students are warmly greeted and farewelled boundaries for appropriate behaviour and consequences are explicitly stated and applied consistently to all students in the same way if students need to be admonished the teacher uses a firm and but calm manner. Shouting and sarcasm has no place in the classroom routines are established and adhered to throughout the year students are treated as individuals student points of view are listened to and acknowledged although not necessarily agreed with no student interrupts the learning of others student behaviour is distinguished from student personality teachers have high expectations of students for both behaviour and achievement. Rewards and Sanctions Commendations are issued to students who have improved their performance or who are achieving a very pleasing standard. Teachers complete the details then the commendations are given to the Head of Year. A positive note written in the contact book is a quick and easy way to send a positive message to parents. The Head of Year may be invited to see a student or class that is working particularly well. If a student is failing to behave appropriately in class, failing to achieve to their potential or failing to complete homework, it is the teacher’s responsibility to work to solve the problem, initially. It is important that the exact problem and action expected by the teacher is clearly communicated to the student. Strategies used by teachers to change student behaviour may include actions such as: seating the student in a different position in class, talking to the student outside class time, writing a note to parents in the contact book keeping a student for part of break or lunch sending a student from the class to the HoD for one lesson in extreme cases of disruption If no progress is made then the HoD must be informed. The HoD will see the student on a regular basis and monitor the situation. If there is still insufficient progress made, then the Head of Year will be informed. 15 Contact Books Contact books are to help students organize their work and for communication with home. Students should have their contact book open on their desk during every lesson. This makes it easy and quick for teachers to write in comments. Contact books should be used by the teacher to write positive or negative comments concerning behaviour, lack of equipment in class, concern about progress. Students should use their contact books to record assessment results, homework and reminders. Time must be given for students to write in their contact books and entries should be checked until students form good habits. If a student fails to have their contact book in class, a missing contact book slip should be completed and passed onto the appropriate Head of Year. 16 The Sultan’s School Science Department 17 Health, Environment and Safety Policy June 2012 Contents 1. General Aims 2. Hazards 3. Risk 4. Risk Assessment 5. Delivery of safety in the laboratory 5.1 Safety signs 5.2 Information 5.3 Laboratories 5.4 Equipment request sheets 5.5 Promoting safety awareness in students 6. Emergency procedures 6.1 Fire 6.2 Spills 6.3 Gas, electrical and water supplies 6.4 Injury 6.5 Reporting procedures 7. Security 7.1 During lessons 7.2 End of lessons 7.3 Break and lunch times 7.4 End of day 8. Equipment and resources 8.1 Safety check list 8.2 Checking and maintenance record 8.3 Fume cupboards 8.4 Electrical equipment 8.5 High pressure equipment 8.6 Chemicals 8.7 Radioactive Materials 8.8 Personal protective equipment 9. Training 10. Miscellaneous items 10.1 Students at special risk 10.2 Visitors 10.3 Substitute teachers 10.4 CLEAPSS 10.5 Review of safety policy and procedures. Appendices 1 to 4 18 1. General Aims It is the duty of all members of the Science Department :(i) To take reasonable care for the health and safety of themselves and other persons who may be affected by their acts or omissions during work. (ii) To seek to minimize any detrimental environmental impact resulting from work of the department. 2. to be familiar with the health, environmental and safety policy of the Department by periodic reference to it to follow its provisions to note any revisions to suggest revisions to the Head of Department if improvements can be seen to cooperate with other members of staff in promoting health, environmental and safety to actively promote health and safety awareness in the students. Hazards A hazard is something with the potential to cause harm, including ill health and injury, to persons, to the environment or to cause damage to property, equipment etc. 3. Risk A risk is the likelihood of a hazard causing harm, and its severity, in practice. 4. Risk Assessment Before any activity undertaken staff must go through a formal risk assessment. 4.1. What are the details of the activity to be undertaken? Even if you are familiar with the activity reconsider the details. If you are unfamiliar with the activity or you are creating a new activity, discuss it with colleagues and refer to related materials on the subject to make sure that you are fully aware of the procedures to be undertaken. 4.2 What are the hazards in this activity? Hazards must be identified. There are numerous sources of information on hazards in the Department, see Appendix 1. If in doubt, please ask. Behaviour of the students must also be considered when identifying a hazard. 4.3 What is the chance of something going wrong? If it can go wrong then, theoretically, one day it will go wrong. However, we must assess the probability that it will go wrong. I suggest four categories:(a) almost certain (b) a good chance that it may 19 (c) unlikely 4.4 (d) very unlikely How serious would it be if something did go wrong? If something did go wrong then we must consider the seriousness of the outcome to persons and equipment. Things foreseen or unforeseen may go wrong, therefore all members of the Department should be aware of the emergency procedures. See the later section on emergency procedures. 4.5 How can the risk(s) be controlled for this activity? Risks may be controlled in numerous ways, for example, by using specialised safety equipment, by making the students aware of the hazards, by changing the organisation of a lesson, by changing or substituting the activity or by canceling the activity altogether. 5. Delivery of safety into the laboratory The Department staff will follow various procedures as routine so that safety measures are delivered in a reasonably consistent manner across the Department. 5.1 Safety signs Responsibility – Lab. Technician Mandatory signs. These are circular in shape. They will be attached to equipment and/or trays. Hazard signs. These are square in shape and will be attached to any hazardous chemical or equipment. Warning signs. These are triangular in shape and warn of hazards and attached to equipment where a warning is required. 5.2 Information Responsibility – Lab. Technician ‘Hazcards’ (CLEAPSS) will be available in all laboratories, the main preparation room and the Science teachers’ staff room. Books on safety will be kept in the main preparation room on an open shelf labelled ‘Safety Information’. The current list is shown in Appendix 1. Posters referring to safety will be displayed in the main preparation room, and where appropriate in the laboratories. 5.3 Laboratories Responsibility – Lab. Technician All laboratories will be equipped with:A copy of “Rules for students during science lessons” displayed on a notice board. Safety equipment such as fire extinguishers, fire blankets and gas ‘cut off’ buttons. A notice giving the position of all the mains “cut of points” Safety goggles – enough for one for each student and member of staff 20 Laboratory coats – enough for one for each student and member of staff One safety screen One set of ‘Hazcards’ One set of ‘Student Safety Sheets’ (CLEAPPS) A permanent display of the main safety signs A set of safety signs with magnetic strips attached A ‘spill kit’ to deal with small spills (including mercury spillage) plus paper towels for general cleanliness. A BDH spillage chart should be displayed on a notice board. Plastic box for broken glass with dust pan and brush for picking up the glass. A copy of “Immediate remedial measures” should be on display near the teachers’ bench. A ‘First aid kit’. 5.4 Equipment Request Sheets Responsibility - Teachers + Lab. Tech During lesson preparation, risk analysis will take place. Any hazards identified by the teacher will be highlighted on the equipment request sheet by circling the appropriate hazard symbol. More explicit notes will also be added if required to give full warning to the laboratory technical staff. See appendix 2 for an example of an equipment request sheet. The laboratory technical staff will review the request sheets and inform the teacher of any further hazards that they feel have been overlooked by the teacher. 5.5 Promoting safety awareness in students. Responsibility - Teachers + Lab. Tech At the beginning of each year each teacher must make sure that their students have a copy of the “Rules for students during science lessons”. See appendix 4. A copy will be displayed in each laboratory and in the “Science Study Guide”. No students will left unsupervised in a laboratory. No behaviour in the laboratory which may constitute a hazard should be tolerated, eg. Eating, drinking, running, boisterous behaviour, high noise levels, etc. Students who persist in such behaviour should be temporarily removed from the practical situation and, after consultation with the Head of Department, may be put on Department surveillance or excluded from laboratory work. Safety signs should be on display in all laboratories. A copy will be included in the “Science Study Guide”. Potential hazards should be highlighted in the introduction to a lesson or section of a lesson. The appropriate safety symbols for the lesson should be displayed at the front of the laboratory using the metal strip and magnetic safety symbols available in each laboratory. The appropriate safety symbols should appear on all practical worksheets. 21 If a material features in the ‘Hazcards’, a copy of the card will be delivered to the laboratory with the materials by the laboratory technician. Students should always be instructed to wear the appropriate safety equipment. Wearing of the equipment is not optional. Teachers should always set a good example and wear the appropriate safety equipment. 6. Emergency procedures All staff should be aware of the emergency procedures and how to implement them if required. 6.1 Fire Science staff will follow the normal school procedures in case of major fires. All Science staff should be familiar with the position of laboratory fire equipment in the room. All Science staff should be trained in the use of laboratory fire equipment to deal with minor blazes in the laboratory 6.2 Spills Spills of any volume that do not give rise to significant quantities of toxic or highly flammable fumes are to be dealt with by teachers or technical staff using the ‘spill kit’ prepared for the purpose. Spills of any volume that do give rise to significant quantities of toxic or highly flammable fumes being present in a room should result in the immediate evacuation of the room by all staff and students. (All laboratories have three doors and all three should remain unlocked during lessons. See Security) 6.3 Gas, electrical and water supplies All staff should be familiar with the position of the points of disconnection of all three supplies in each laboratory. 6.4 Injury If an injury does occur all Science staff should take remedial action until the nurse arrives. This is set out in “Immediate remedial measures” See Appendix 4. A ‘First aid kit’ is available in each laboratory; however, if the injury is more than very minor, the school nurse should be called. If the nurse is unavailable then please contact Trevor Blackman. 6.5 Reporting procedures All records will be held by the laboratory technician in the main preparation room. All injuries to students or staff, however minor, should be recorded in the “Injuries Record”. All breakages should be recorded in the “Breakages Record”. All dangerous or potentially dangerous incidences should be recorded in the “Incidents Record”. 22 A “Checking and Maintenance Record” will be kept by the laboratory technicians. 7. Security 7.1 During lessons all three exit doors from each laboratory will be unlocked. 7.2 At the end of each lesson the teacher leaving the room will lock all the laboratory doors, unless another teacher is to immediately take over the laboratory. 7.3 At break and lunch times, each laboratory and the preparation room should be locked by the last member of staff to leave each room. 7.4 At the end of the day the technical staff are responsible for closing down each laboratory and leaving the laboratories in a safe condition. This includes disconnecting the gas supply. 8. 8.1 8.2 Equipment and resources A safety check list will be produced and updated by the technical staff. A “Checking and Maintenance Record” will be kept by the laboratory technicians. The technical staff are responsible for making sure that all the necessary checking and maintenance of equipment is carried out. 8.3 Fume cupboards will be checked annually. 8.4 All electrical items will be checked annually. 8.5 Autoclaves, pressure cookers and model steam engines will be checked annually. 8.6 The safe storage of chemicals and radioactive isotopes will be continuously monitored; however, an overall check will be made every six months. The Head of Chemistry must be consulted on the safe storage of chemicals. The Head of Physics must be consulted on the safe storage of radioactive isotopes. These must be collected, signed out and returned by the individusl teacher. 8.7 Personal protective equipment will be continuously monitored; however, an overall check will be made every six months. 9. Training 9.1 All staff should be trained in the use of safety equipment in the laboratories. 9.2 All staff should be made aware of the method and position of disconnection equipment for the three main services in each laboratory. 9.3 9.4 Cleaners and maintenance staff should be made aware of hazards in the laboratories and in the use of safety equipment and disconnection of the three main services. Annual update and review of training is the responsibility of the Head of Department. 10. Miscellaneous items 23 10.1 Students at special risk The administration must inform members of the Science Department of any student who may be at risk in a laboratory because of medical (eg. epileptic fits) or other problems. 10.2 Visitors Visitors to the Department must always be supervised by a member of the Department. 10.3 Substitute teachers Only members of the Science Department teaching staff may supervise students in Science laboratories. 10.4 10.5 CLEAPSS – Consortium of Local Education Authorities for the Provision of Science Services (a) The Science Department will be a member of CLEAPSS (b) The laboratory technicians will circulate to all staff the CLEAPSS documents as they arrive in school and will then file the documents for future reference. (c) The laboratory technicians will maintain and update the CLEAPSS safety files. (d) An updated CLEAPPS CD ROM is installed in ‘Science Shared Resources’ on the Intranet. Review of safety policy and procedures Safety procedures will be under constant review. Any member of staff who can see a potential improvement in the present procedures should report it to the Head of Department. An annual review of safety policy and procedures will take place within the first six weeks of each academic year. A report will be written by the Head of Department and given to the Principal stating the state of the HSE procedures and any modifications. 24 Appendix 1 Safety Information This information is stored in the main preparation room on an open shelf labelled ‘Safety Information’, with the exception of Hazcards which are available in all laboratories. 1. CLEAPPS CD ROM: Stored in CLEAPPS folder in Science Admin filing cabinet 2. CLEAPSS Laboratory Handbook (Consortium of Local Education Authorities for the Provision of Science Services, UK) 3. Hazcards (CLEAPSS) 4. CLEAPSS Student Safety Sheets 25 Appendix 2 Equipment request sheet These sheets are to be completed for each lesson when equipment is required. Folders for each day are attached to the notice board in the main preparation room. The sheets are posted in the folder for day that the equipment is needed. The sheets should normally be submitted one week in advance of the lesson. Date: Class: Science Department-Equipment Request Bio-hazard AJO MPO AQR Toxic EJT JMO SRA Corrosive SAT SUN MON TUE Irritant Y3 Y7 G15 A10 Harmful 0+1 1+2 3+4 5&6/7 Flammable Y4 TBL IBE OPR KTR RNO WED A11 A12 A21 8&9 A22 Equipment Required Electrical Laser Oxidising Explosive Radiation Protective Clothing Eye-Protection Waste Materials Hot Apparatus Other Comments: 26 Appendix 3 IMMEDIATE REMEDIAL MEASURES for Science Staff What Science Staff should do while waiting for first aid. The First Aid Regulations do not necessarily require there to be a qualified first aider among science staff, yet this is clearly desirable. Nevertheless, all staff have a duty to carry out remedial measures immediately while waiting for first aid or professional medical treatment. The following advice covers common laboratory accidents and is intended as a supplement to any local guidance on dealing with non-laboratory events, eg, epileptic fits. Chemical splashes in the eye Immediately wash the eye under running water from a tap for at least 10 minutes and for much longer in the case of alkalis. The flow should be slow and eyelids should be held back. Afterwards, the casualty should be taken to hospital (with irrigation continuing during the journey for an alkali in the eye). More advice on washing is given in [the CLEAPSS Laboratory Handbook Section 3], [Safeguards in the School Laboratory p107]. Chemical splashes on the skin Wash the skin for 5 minutes or until all traces of the chemical have disappeared. Remove clothing as necessary. If the chemical adheres to the skin, wash gently with soap. Chemicals in the mouth, perhaps swallowed Do no more than wash out the casualty’s mouth. After any treatment by the first aider, the casualty should be taken to hospital. Sometimes attempts are made to administer an 'anti-dote'. This is likely to do more harm than good and should not be attempted. Burns Cool under gently running water until first aid arrives. Toxic gas Sit the casualty down in the fresh air. Hair on fire Smother with a cloth. Clothing on fire Smother by pushing the casualty to the ground, flames on top. Spread a thick cloth or garment on top if necessary. A fire blanket is ideal but use only if very close by. Electric shock Taking care for your own safety, break contact by switching off or pulling out the plug. If it is necessary to move the casualty clear, use a broom handle or wooden window pole or wear rubber gloves. If casualty is unconscious, check that airways are clear and begin artificial ventilation if necessary. 27 Severe cuts Lower the casualty to the floor and raise the wound as high as possible. Apply pressure on or as close to the cut as possible, using fingers or a pad of cloth. Protect yourself from contamination by blood. Leave any embedded large bodies and press round them. 28 Appendix 4 Rules for students during science lessons 1. You must not enter a laboratory unless instructed to do so by a teacher. 2. You must not do anything with equipment or materials unless told to do so by a teacher. You must follow instructions precisely. 3. You must wear eye protection when told to do so and keep it on until told to take it off when all practical work, including clearing away, is finished. 4. When instructed to use a Bunsen burner, make sure that hair, scarves, ties etc. are tied back or tucked in to keep them well away from the flame. 5. When working with liquids, normally stand up; then you can move out of the way quickly if there is a spill. 6. Never taste anything or put anything in your mouth when in the laboratory unless your teacher tells you to do so. This includes sweets, fingers and pencils which might have picked up dangerous chemicals from the bench. 7 If small amounts of chemicals or microbiological cultures get on your hands or any other part of the body, wash them off. Wash your hands after work with chemicals or with animal or vegetable matter. 8 Put waste solids in the correct bin, never in the sink. 9. Report any accident to the teacher. This includes burns or cuts and chemicals in the mouth, the eyes or on the skin. 10.Keep your bench clean and tidy, with bags put in a place where people will not trip over them. Wipe up small splashes with a paper towel and report bigger ones to the teacher. 29 30 31 Lessons 1) Go to your lessons well prepared. Here is a checklist of some of the things that you may need: Homework to be handed in Textbook Folder and paper Pencil Pen Ruler Calculator Protractor, compass etc. 2) During the lesson: Listen carefully to the teacher. He or she has important information for you. Don’t miss anything Remember: “ It’s a live show”. textbook. You will only see and hear things once. Think. Ask questions if you are not sure. Make notes. The teacher may give you a worksheet or tell you to look at the 3) At the end of the lesson: Make a note of all the work you have to do and the date that you have to finish it. 32 Homework You should do this work alone and without the direct help of your teacher. You will find it difficult at times and you will make mistakes but this is a very important part of learning. When your teacher corrects your homework he or she will see your difficulties and decide how to help you. COPYING KILLS LEARNING! If you copy from another student, your teacher will not know about your difficulties until it is too late to give you any help. Points to help you do your homework: Set a regular time each day for homework and keep to it. Work in a quiet place, with a good writing surface and good light. Promise yourself something you like at the end of homework. If your homework session is 2 hours or more have a break and a drink when you have finished half of it. If you finish all the work set by teachers, complete your time by reviewing, organising or rewriting rough notes. Read through the section of the textbook for the classes that day or that week. Don’t leave all the set homework until the night before you need to give it to the teacher. DON’T work too long at night. DON’T play loud music or watch TV. DON’T make countless drinks and snacks. DON’T ring up a friend as soon as you have a problem or you are bored. 33 Written Work A lot of your work will be written, so you must present it clearly. Write the date, your full name and your group on work you give to your teacher. Put all your written work in your file in date order. Notes Underline main and side headings. Highlight equations and definitions to give your notes “life” and If you write rough notes during a lesson, write them out clearly for homework, even if your teacher doesn’t tell you to do this. Answers to questions You do not normally have copy out questions before you try to answer them but give a reference, e.g. p65 No. 1. You should normally answer in full sentences. You can use diagrams to illustrate answers and if your teacher asks for diagrams, they are essential. e.g. Q1. How many legs does a spider have? Answer: 8 A good answer (without the question) would be: A spider has eight legs. A poor answer would be: 8 Remember: You will need your answers for review later 34 Errors in Measurements There are two types of error which occur when you take readings:Systematic errors These are errors, which may be built into the particular measuring system that you are using or the way in which the measurements are taken. For example, if a balance reads 20g when no mass is on it then all the readings taken during the experiment will be 20g too large. This is known as a zero error. If someone always reads the pointer on a scale from the right hand side then a constant parallax error will change the reading. Heat losses may occur whilst you are performing an experiment to find the specific heat capacity of a substance. This will reduce the value of the actual readings you take. Systematic errors cannot be reduced by taking many readings and averaging. To reduce systematic errors some change must be made in the way the readings are taken or used. In the examples above the balance should be zeroed at the start of the experiment or the zero error subtracted from each reading. The student should read from directly in front of the scale to avoid parallax errors. In heat experiments, the apparatus needs to be improved to reduce heat loss or there needs to be some mathematical method for estimating heat loss. Random errors These are errors which occur as deviations from the true value. For example, if a scale is read several times, the readings may vary about the true value as shown in the diagram. The vertical lines represent the readings. The effect of random errors may be reduced by taking a number of readings and taking the average or similar techniques. Random errors 88 89 90 cm3 35 Answers to Numerical Problems You need to show ALL of your working (calculation). (Most marks are awarded for the working) You always need to think about units. State any equation you are going to use. Substitute the correct values into the equation. Work out the arithmetic. Write down a full statement of the answer with units. Example: Question: Answer: 0.1 m 3 of aluminium has a mass of 270 kg Calculate the density of aluminium. Density = mass volume Density of aluminium = 270kg 0.1m 3 = 2700 kg/m3 Density of aluminium = 2700 kg/m3 or 2700 kgm-3 36 Writing up Practical Work Practical work is a major part of your study. Practical work may be demonstrations or class experiments, and may involve one drawing or several observations and/or measurements. Any experimental report your teacher asks you to write should have the following format, but not necessarily in this order: Title/aim Diagram Diagrams can often reduce the number of words you need to write. They MUST BE: in pencil fully labelled simple outlines beaker xxxxxxx large water gauze tripod HEAT Method/Procedure Simple short sentences about what you did. This could be in point form, i.e. 1) 2) etc... Results/Readings/Observations This is a record of the measurements or observations made. Tables are often used. Calculations Use your measurements to calculate quantities. This section can include graphs. Conclusion/Discussion back Evaluation What you have learned or found out. You should refer to the aim. Suggest improvements that could be made to the experiment. Discuss experimental errors. 37 Graphs Graphs are often used in science to present results and show relationships between things. Usually, they will be line graphs but bar charts, histograms and pie charts can also be used. Make sure you have a sharp pencil Drawing a line graph Readings From your table of results: Volume/cm3 0 10 20 30 40 50 60 Height/cm 0 2.1 4 6.1 7.9 10 12 Axes Look at your readings and choose a scale so that your final graph will use most of the graph paper. Draw the lines in pencil. Which way round do the axes go? The independent variable (the one changed by you) should go on the x or y=0 axis. The dependent variable (the one that changes as a result of what you did) should go on the y or x=0 axis. 38 Each axis should have THREE things on it: 1) the scale (this should be even or have equal divisions); USE A PENCIL 2) what you are plotting, e.g. volume 3) the units e.g. cm3 2) & 3) should be written as volume/cm3 Plotting the points In science, the points you plot generally come from experiments where measurements have been taken. Measurements are all subject to error. Therefore, the dot you mark on the graph paper may not be exact. To show this, we can use one of two methods of plotting a point: or . Drawing the line a) A straight line graph. (This usually show a simple relationship between the two quantities.) 39 DO NOT JUST JOIN THE POINTS If the points seem to lie in a general straight line, then TAKE A RULER and draw a straight line through the centre of all the points. A line of best fit can be drawn when one or two of the points fall a little above or below. * Now use results on the draw a graph Hint: Keep your hand on the inside of the curve Check that the curve is smooth by holding it up to your eye and looking along it. It should be smooth like a motorway not with kinks and bumps like a wadi track! the table of previous page to straight line b) A curved graph. DO NOT JUST JOIN THE POINTS Draw a smooth curve with a single thin line. Again, this may go through all the points or it may be a line of best fit. * Use the results in table below to draw a curved graph Volume/m3 0.25 0.5 1 1.5 2 2.5 3 3.5 4 Pressure/kPa 8.5 7 5 3.5 3 2 1.7 1.5 1 c) Special graphs.Sometimes in Biology, you may be asked to join up the points e.g. when showing the stages in insect or human growth. 40 Review for tests and exams You learn a lot of things without knowing that you have learned them. You should learn from experience in lessons how to: tackle scientific problems observe and measure test ideas look for patterns predict what will happen write up an experiment plot graphs However, there are still a certain number of basic facts that need to learned before you enter the exam room. Here is one method of remembering that works for most people, and it can work for you if you make the effort. Personal Review Cards At the end of a topic make a review card on a piece of card about the size of a postcard. Look through your work and make a note of all the points covered. These may be definitions, equations, diagrams, observations or even example calculations. Now write these on your card in an interesting way and keep them brief: Underline certain things in colour. Put stars round an equation. Use a highlighter pen. Stick on pictures. 41 You could use mnemonics to remember the initial letters of a list of things. (A mnemonic is a special sentence, or maybe a song or poem, that helps you to remember things.) Example King Philip Came Over From Germany Singing Songs Kingdom Phylum Class Order Family Genus Species Sub-species There are two golden rules when making cards: 1) Every card should be DIFFERENT from all the others; try different coloured inks. (If you spill coffee on one, leave it, you’ll never forget that one!) 2) NEVER write anything you don’t understand. (See your teacher to sort out any difficulties at an early stage) How to use your review cards: Take your first card and put it in a safe place. When you finish the next topic, take out your first card and read it through carefully before you make your next card. Continue to do this for all the following topics. By the end of the year you could have 20 or more cards for each subject. If you have worked on them correctly you should be able to remember all the details. You will have a “photograph” in your mind. Bring your cards to school and get your friends to test you. They will be amazed at your powers! 42 Reviewing before tests and exams: If you have already made review cards through the year, half the work is done. Whatever you have done there is one golden rule for reviewing: REVIEWING MUST BE ACTIVE Here are some guidelines: Read through a topic, using your notes, experiment write ups and text book; then, without looking, write down the main points. Ask a friend/relative to quiz you after each topic. Try to answer old homework questions and compare with your original answer. Try to make up your own questions on a topic. Use review questions in your text book. Look at a diagram, close the book; try to draw it and label it. Make a note of things that you are not sure about so that you can ask your teacher. Don’t leave this until the morning before your exam! There are many ways to review. You must find what works for you but it must be active; simply reading through a textbook hoping you will remember everything won’t work. Your mind will just go on to other things. Get plenty of sleep; don’t stay up all night. 43 Quizzes, Tests and Examinations 1) Arrive in good time with all the necessary equipment. 2) Answers should be written in ink. 3) Read the instructions on the question paper carefully. No extra marks are awarded for answering more questions than you need to. 4) Divide your time carefully, do not spend disproportionate amounts of time on one question. 5) Try to answer all the required questions. if you get stuck on one part leave it and come back to it at the end if you have time. 6) Marks for each part of a question are given in brackets, use this as a guide to how much time you spend on it or detail required. 7) There are no awards given to the person who finishes first. 8) When you are finished, check your answers through very carefully. 9) Check units. Every missing unit or incorrect unit will lose one mark in the examination! 44 Definitions of Action Verbs commonly used in Examinations and Tests Define State List Draw Measure Estimate Outline Describe Calculate Identify Apply Compare Contrast Annotate Suggest Discuss Explain Deduce Predict Evaluate Design Determine Analyse Define Defend (support) Prove Give a precise meaning of a word or phrase as concisely as possible. Give a specific name or other brief answer. No supporting argument or calculation is necessary. Give a sequence of names or other brief answers with no elaboration (extra information). Each name or answer clearly distinguished from the others. The question may tell you the number of items in the list. Represent by means of pencil lines. Add labels unless told not to do so. Find a quantity and state it using a number and an SI unit. Find the most likely value for an unknown quantity, based on information provided and scientific knowledge. Give a brief account or summary, including essential information only. Give a detailed account, including all relevant information. Find an answer using mathematical methods. Show the working unless instructed not to do so. Find an answer from a number of possibilities. Use an idea, equation, principle, theory, or law in a new situation. Give an account of similarities and differences between two (or more) items, referring to both (all) of them throughout. Comparisons can be given using a table. Give an account of the differences between two or more items Add brief notes to a diagram, drawing or graph. Propose a hypothesis or other possible answer. Give an account including, where possible, a range of arguments, assessments of relative importance of various factors or comparison of alternative hypotheses. Give a clear account including causes, reasons or mechanisms. Reach a conclusion from the information given. Give an expected result. Assess the implications and limitations. Produce a plan, object, simulation or model. Find the only possible answer. Interpret data to reach conclusions. Give the meaning of a term or concept. Argue a position, based on a suitable selection of data. Show the truth of a proposition using deductive logic. Good Luck with your Studies ! 45 Rules for Students during Science Lessons 1) You must not enter a laboratory unless a teacher tells you that you can. 2) You must not do anything with equipment or materials unless a teacher tells you to do it. You must follow instructions exactly. 3) You must wear eye protection when a teacher tells you to wear it, and you must keep it on until a teacher tells you to take it off when all practical work, including clearing away, is finished. 4) When a teacher tell you to use a Bunsen burner, make sure that your hair, scarves, ties etc. are tied back or tucked in to keep them well away from the flame. 5) When you are working with liquids, you must normally stand up. (If you do this, you can move out of the way quickly if the liquid spills.) 6) Never taste anything or put anything in your mouth when you are in the laboratory unless your teacher tells you to do so. This includes sweets, fingers and pencils; they might have picked up dangerous chemicals from the bench. 7) If small amounts of chemicals or microbiological cultures get on your hands or any other part of your body, wash them off. Wash your hands after working with chemicals, or with animal or vegetable matter. 8) Put waste solids in the correct bin, never in the sink. 9) Report any accident to the teacher. This includes burns or cuts and chemicals in the mouth, the eyes or on the skin. 10) Keep your bench clean and tidy, with bags put in a place where people will not trip over them. Wipe up small splashes with a paper towel and report bigger ones to the teacher. 46 Science Department Minutes Attendees: Trevor(chair), Jos, Ian, Trevor D, Alan, Mark, Ha Harry, Jacqueline, Emmajane, John, Adnan, Ruth Ruth. Apologies: Glenn 22/01/2012 Items Action 1. Welcome A very warm welcome to John and all the very best for your new career here at The Sultan’s School 2. Technician’s Report Our team are very busy stocktaking at the moment prior to updating our inventory. Heads of subject need to be starting to think about the orders for 2012-2013. Book orders could be prepared now with equipment and consumables when inventory complete. Trevor to enquire as to the state of play regarding students purchasing their own books. Year 7 completing Electricity and Magnetism. Going on to Differences & Classification Year 8 completing Life Support. Going on to Forces Year 9 completing Metal Reactions. Going on to Light & Sound. All Teachers’ Guides available. Tests being printed. Please liaise with team members as to best time to set the tests and who will be marking them. 3. Key Stage 3 Update 4. IGCSE Update It was universally agreed that on the whole it appears that we have a highly motivated Year 11 cohort. Hopefully the mocks will confirm this. All on target 5. IB Update Thanks to Departments for putting in significant extra time; weekends / evenings to ensure curriculum coverage and IA completion. The 3rd editions of the Question Banks are shortly available. Trevor to order Environmental Systems Question Bank arrived The deadline for submitting IA sample sets with necessary forms to IB coordinator is March 21st. Most papers now with Brian Stewart please check them and fill in cover sheets Decision as to who sits Core / Extended to be made immediately after the IGCSE mocks Trevor to draft letter to parents 6. 7. Mock Examinations IB Induction Monday 30th January during the day for students. Tuesday 31st January during the evening for parents. In the light of parents and students being attracted to the proposed A-level centre and other institutions offering the Thanawiyam’a these sessions are aimed at positively promoting the IB at The Sultan’s School. These key points were proposed: Eli to give a presentation 47 Adnan to discuss university implications Display Boards to be set up by Departments and manned by current IB students. CAS students allocated to a Department? CAS students to meet and greet Parent of 4 previous IB students to speak IB ‘taster’ session after the exams Future innovations: 8. 9. Group 4 Project Graduation 10. AOB Students issued with Ipads /tablet computers loaded with relevant apps. Dedicated IB Study Centre to be completed. Please send photos for the display to Hayley-anne soonest Sudheer will take photos if you book him Two full days; the Sunday and Monday last week of term Need to start thinking of possible investigations Same as previous years? Interesting possible investigation on the properties of camel’s milk: Ruth has arranged a visiting speaker Do we wish to have a Science Prize / Prizes? .One per year group? Prize for initiative in project work? Please let me know your ideas. Alan suggested that we actively regularly promote our undoubted strengths to our students particularly Year 11. Good idea! Trevor 22nd January 2012. 48 SCIENCE EXAMINATION REPORT 2008 Examination results analysis – Physics 2008 IB Grades 7 0 0 0 0 0 0 Higher Standard Total 2008 % 2007 2007 % 6 5 1 6 18 4 9 5 4 0 4 12 3 7 4 6 3 9 27 11 26 3 3 8 11 33 17 40 2 0 3 3 9 8 19 1 0 0 0 0 0 0 A very pleasing improvement on last year’s results – 57% achieving 4 or above, compared to 42% the previous year, and double the proportion at 5 or above. Still some concern over 3 students scoring 2. Component Analysis Higher Level 7 P1 P2 P3 Practical 1 0 1 1 6 1 3 4 4 5 3 5 4 8 4 5 4 3 4 3 6 3 4 0 2 0 2 1 0 1 0 0 0 0 Higher level students clearly found Paper 2 to be the hardest scoring significantly lower grades on this. Interestingly, P3 produced the best mark for many candidates. This is the Option Topic paper, and although the material covered has a high intellectual demand, students are able to focus on narrower sections of the syllabus. Standard Level 7 P1 0 P2 0 P3 1 Practical 0 6 0 0 0 0 5 1 1 1 8 4 0 3 2 6 3 9 4 4 2 2 6 7 7 0 1 0 1 1 0 In contrast to the Higher level, Paper 3 proved more difficult, although 1 student scored a 7. Comparison to Predictions Correct prediction 17 51% Actual +1 Actual +2 Actual -1 6 18% 1 0% 9 27% 49 Virtually all students were within 1 grade of prediction. 1 student who scored 2 grades above prediction made a strategic choice early in Year 2 to switch from Higher to Standard. She was much more suited to the demands at this level, and hard work on her part brought well-deserved success. IGCSE Grades Predicted 0 1 3 8 12 16 12 3 43 G F E D C B A A* A*-C Actual 0 0 2 5 14 18 11 4 47 % 0 0 4 9 26 33 20 7 Predicted Distribution 20 15 Predict ed 10 Act ual 5 0 G F E D C B A @ Comparison to Predictions Correct prediction 28 52% Actual +1 Actual +2 Actual -1 12 22% 5 9% 9 17% Overall, students achieved a Physics grade approx 0.4 of a grade higher than their average for all their other subjects. This was the first year of examination for the Edexcel syllabus. 83% of students achieved or exceeded their predictions. 50 Biology IB EXAM RESULT ANALYSIS May 2008 exam This group was unusual in that there was a small group of 5 HL candidates and a larger group of 13 SL candidates. Unfortunately the two were coalesced into a composite class from day 1 (but there were 19 in total. one chap did not return for IB2). BIOLOGY HL Grade 6 5 2008 3 1 2007 2 5 2007 Global Average ie for the world 4 1 2 3 2 2 1 mean 5.4 4.4 4.1 2008 Actual grades vs Predicted Grades: 3 ‘spot-on’, 2 were predicted one grade lower Comment: This was a small but able group who were severely disadvantaged by being taught in a composite SL-HL class. Pleasing results. BIOLOGY SL Grade 6 5 4 2008 4 6 2007 3 5 2007 Global Average ie for the world 3 3 1 2 - mean 4.1 4.2 4.3 2008 Actual vs Predicted Grades: 6 spot on, 7 were predicted one grade lower Given the poor caliber of the SL class, I am extremely pleased with their results 51 COMPONENT BREAKDOWN (Grades ranked in order) HL BIOLOGY- 5 students Paper 1 m/choice 6 Paper 2 Bulk 7 Paper 3 Option 6 Practical Work 6 SL BIOLOGY- 13 students P1 7 5 5 5 P2 6 5 5 5 P3 6 5 5 4 PW 5 4 4 4 6 7 5 6 5 7 5 4 4 5 4 4 5 5 5 4 3 4 4 4 3 4 4 4 3 4 4 3 3 4 4 3 3 4 4 3 3 4 4 3 3 3 4 3 3 3 4 3 3 2 3 3 Comments HL (and SL) showed a particularly strong performance in Paper 2, which is pleasing as this paper is worth the most in the weighting. SL did surprisingly well in Paper 3. It would appear that paper 1 (the Multiple Choice) is the greatest area of weakness and an obvious target for improving performance with the next group. Perhaps our students rely too much upon recognizing questions that they have seen before (in school test situations) rather than considering the problem and ruling out the red herrings. Paul Cooper told me that Economics has dropped the Multiple Choice Exam as it was a simple test of English comprehension rather than subject understanding for International students. That may be a factor. More data is needed to make an evaluation. The Practical Work grades were very satisfactory this year. By this I mean that they were not drastically lower than the other subjects as in previous years and is our sientists’ annual gripe with IB Moderation. Perhaps my altered strategy of targeting a few practicals and marking only these ones has paid off. Or maybe we just happened to get a ‘better’ marker than last year. HL- 3 of 5 students gained Grade 7s in Paper 2 SL- 1 of 13 gained a Grade 7 in Paper 1 (Omar Busaidi) SL- 5 of 13 gained a 5 or 6 in Paper 2 Grade 7 still eludes Biology and is likely to do so given the vagaries of Internal Assessment marking. At least the 7s are appearing in the exam components of the more able. Alla Lawati was the top student, he achieved a Grade 4 in Biology Internal Assessment (which is fair and correct) as he had problems managing workloads and deadlines. I hope that he has learned his lesson in IB and achieves potential at Uni. Conclusion I am very happy with this set of results given the dire quality of the SL candidates (except for Omar Busaidi) and the HL-SL combination retarding progress for the HL students. 52 IB EXAM RESULT ANALYSIS Chemistry May 2008 exam This group was unusual in that there was a small group of 5 HL candidates and a larger group of 13 SL candidates. Unfortunately the two were coalesced into a composite class from day 1 (but there were 19 in total. one chap did not return for IB2). CHEMISTRY HL Grade 7 6 5 2008 1 7 5 2007 5 5 2007 Global Average ie for the world 4 10 9 3 2 5 2 1 mean 4.8 4.3 4.5 2008 Actual grades vs Predicted Grades: 11 ‘spot-on’, 6 were predicted one grade lower 7 were predicted 1 grade higher 1 was predicted 2 grades lower Comment: Pleasing significant improvement on last years' results (better cohort) CHEMISTRY SL Grade 7 6 5 2008 1 2 2007 2 1 2007 Global Average ie for the world 4 3 5 3 3 2 2 1 2 mean 4.2 3.9 3.9 2008 Actual vs Predicted Grades: 7 spot on, 2 were predicted one grade lower 1 was predicted 2 grades lower Comment: Again a pleasing improvement. 53 COMPONENT BREAKDOWN (Grades ranked in order) HL CHEMISTRY- 25 students Grades 7 6 Paper 1 m/choice 2 2 Paper 2 Structured 7 Paper 3 Option 1 5 Practical Work 8 13 SL CHEMISTRY- 10 students Grades 7 6 5 4 P1 1 1 1 2 P2 2 1 2 P3 PW 2 5 2 1 5 3 8 3 3 3 5 2 4 4 3 7 1 2 2 3 13 5 5 - 1 1 2 1 2 4 - Means 3.92 4.12 4.04 6.12! Means 4.1 3.8 4.0 5.5 Comments At Higher Level the multiple choice paper proved to the weakest. I considered this year's paper to be the most difficult of those set in recent years. The structured paper has a significantly higher weighting than the other components and has the best results of the theory papers. The most surprising feature here is the grade point average for the Internal Coursework component. The means for both Higher and Standard Levels are way above last years', (almost exactly 4). Whilst it is pleasing that the students did very well in this component, it does underline the total subjectivity of the external moderation procedure: Exactly the same Practical Scheme of Work as last year. Exactly the same internal assessors as last year. All Chemistry students were moderated down by 4 marks (out of 48) this year. Last year students were moderated down between 13!! and 4 marks. The moderation procedure is inconsistent and incomprehensible. 54 IGCSE Chemistry Exam Performance Analysis May 2008 Comparison of 2008 with 2007 ( Numbers taking Core in Parentheses). For Core C is the top grade. A* A B C D E F G Total 2008 3 10 12 3(+6) 1+(5) 1+(2) (1) 62 2007 8 11 10 17 (+1) 4(+5) 4 (+6) 0+(7) 0 54 A*-C Excluding Core 89.36% A*-C Including Core 69.35% 89.92% 70.40% Comments Whilst the percentage A*-C grades is slightly lower than last year, these results greatly exceed our expectations based on this cohort’s Mock Examination results. We predicted that 30 students would achieve A*-C based on their mocks, giving an average of 48%. As a result the great majority of students achieved their predicted grades or better with very few underachieving. The 5 students scoring below a C in the Extended paper were advised that Core might be the better option. The student scoring the F grade was allowed to take the Extended option after ‘tearful’ representations in the company of her mother! The reasons for the students exceeding our expectations would be: A rigorous and structured emphasis on past paper exercises well in advance of the examinations. The students themselves working much harder after the Mock Examinations, (realizing the standard required). Continued choice of the Alternative to Practical paper option. Note: The A* to C % could be a false statistic in that we (the Sciences) enter all of those students with any chance of achieving a Grade C into the Extended option. Thus it is rare for students to achieve this from the Core option, (the student who achieved this this year requested to do the core, even though he was averaging a solid Grade C). 55 SCIENCE EXAMINATIONS REPORT 2009 IB EXAM RESULT ANALYSIS Chemistry CHEMISTRY HL Grade 7 6 5 2009 6 6 2008 1 7 5 2007 5 5 2007 Global Average ie for the world 4 8 10 9 May 2009 exam 3 1 2 5 2 1 mean 4.9 4.8 4.3 4.5 2009 Actual grades vs Predicted Grades: 9 ‘spot-on’ 9 were predicted 1 grade higher 1 was predicted 2 grades higher Comment: Slight improvement on last years' results, disappointing that there were no Grade 7s. (See comment below) CHEMISTRY SL Grade 7 6 5 2009 1 3 2008 1 2 2007 2 1 2007 Global Average ie for the world 4 3 3 5 3 2 3 2 2 5 1 2 2009 Actual vs Predicted Grades: 8 spot on, 3 were predicted one grade lower 2 were predicted 1 grades higher 1 was predicted 2 grades higher mean 3.5 4.2 3.9 3.9 Comment: Significantly lower grade point average, but results pretty much as predicted. Very poor cohort, a number of these students would have been guided imto Environmental Systems had it been available. 1 2008 COMPONENT BREAKDOWN (Grades ranked in order) HL CHEMISTRY- 25 students Grades 7 6 Paper 1 m/choice 2 2 Paper 2 Structured 7 Paper 3 Option 1 5 Practical Work 8 13 SL CHEMISTRY- 10 students Grades 7 6 5 4 P1 1 1 1 2 P2 2 1 2 P3 PW 2 5 2 1 5 3 8 3 3 3 5 2 4 4 3 7 1 2 2 3 13 5 5 - 1 1 2 1 2 4 - Means 3.92 4.12 4.04 6.12! Means 4.1 3.8 4.0 5.5 2008 Comments At Higher Level the multiple choice paper proved to the weakest. I considered this year's paper to be the most difficult of those set in recent years. The structured paper has a significantly higher weighting than the other components and has the best results of the theory papers. The most surprising feature here is the grade point average for the Internal Coursework component. The means for both Higher and Standard Levels are way above last years', (almost exactly 4). Whilst it is pleasing that the students did very well in this component, it does underline the total subjectivity of the external moderation procedure: Exactly the same Practical Scheme of Work as last year. Exactly the same internal assessors as last year. All Chemistry students were moderated down by 4 marks (out of 48) this year. Last year students were moderated down between 13!! and 4 marks. The moderation procedure is inconsistent and incomprehensible. 2 2009 COMPONENT BREAKDOWN (Grades ranked in order) HL CHEMISTRY- 22 students Grades 7 6 Paper 1 m/choice 2 8 Paper 2 Structured 3 7 Paper 3 Option 1 Practical Work 6 SL CHEMISTRY- 14 students Grades 7 6 5 4 P1 1 3 2 P2 1 2 2 3 P3 1 3 PW 4 8 5 4 7 2 12 3 6 1 2 4 8 3 5 3 2 2 5 2 - 3 2 5 - 1 1 7 - 2 8 - Means 5.18 5.27 3.05 4.91 Means 3.64 3.64 2.07 4.14 2009 Comments At Higher Level the multiple choice paper and the structured paper had significantly higher average than last years’. The averages for the Higher and Standard Level options paper were significantly lower than last years’ for the same options chosen. These were the most difficult papers as reflected in the grade boundaries: 26/40 for a grade 7 at standard level, 36/50 for a grade 7 at higher level. Perhaps we need to look closely at other option topics to ascertain their suitability for our students. Again the Practical Work moderation procedure remains a mystery. Whilst the grade point averages are good at Higher Level and satisfactory at Standard Level, they are significantly lower than last years’, with students being moderated down between 11 and 5 marks! (Similar to 2 years ago, but totally different from last year, for the same practical scheme of work and the same assessors!). None of the current Science staff have been trained in the new practical assessment / grading procedure. Perhaps this needs to be addressed. 3 IGCSE Chemistry Exam Performance Analysis May 2009 Comparison of 2008 with 2007 ( Numbers taking Core in Parentheses). For Core C is the top grade. A* A B C D E F G 2009 4 11 13 3 10 12 6 (+4) 3(+6) 2 (+1) 1+(5) 0 2008 1+(2) 2007 8 11 10 19 (+1) 17 (+1) 4(+5) 4 (+6) 0+(7) 0 Total 0 A*-C Excluding Core 87.27% A*-C Including Core 78.68% (1) 62 89.36% 69.35% 54 89.92% 70.40% Comments A fantastic improvement on the previous year’s results and again a significant improvement on our predicted grades for this cohort: Predicted A*-C grades was 65%. A number of factors probably account for this including: Extensive past paper review scheme after the mock examinations. A superb revision scheme posted on the network by Adnan. The 13 D and E grades were all predicted as being so. 4 Examination results analysis – Physics 2009 IB Grades 7 1 1 2 4 0 0 Higher Standard Total 2009 % 2008 2008 % 6 3 1 4 9 6 18 5 4 6 10 22 4 12 4 5 11 16 35 9 27 3 1 12 13 28 11 33 2 0 1 1 2 3 9 1 0 0 0 0 0 0 Higher Level Grade Average was 4.86, approx ¼ of a grade better than world average of 4.63. Standard Level Grade Average was 3.91, slightly below world average of 4.09. A continued very pleasing improvement on last year’s results – 70% achieving 4 or above compared to 57% the previous year and 42% in 2007. Only 1 student scored grade 2, and he had been flagged as a cause for concern throughout the course. Component Analysis Higher Level 7 P1 P2 P3 Practical 0 1 1 4 6 5 3 2 2 5 3 6 4 6 4 1 1 2 2 3 4 3 5 0 2 1 0 0 0 1 0 0 0 0 P2 was the paper producing the weakest grades, but much better than last year. Again some good results on Paper 3, the option topics. Of interest is that no students scored a 7 on Paper 1. This is a multiple-choice paper with questions of fiendish complexity – students expect this style of paper to be easy and are often surprised at how difficult the questions are. 5 Standard Level 7 P1 1 P2 0 P3 1 Practical 0 6 1 2 1 4 5 6 7 3 17 4 7 11 5 8 3 9 11 6 3 2 6 1 9 0 1 2 0 7 0 In contrast to the Higher level, Paper 3 proved more difficult, although 1 student scored a 7. Comparison to Predictions Correct prediction 20 43% Actual +1 Actual +2 Actual -1 15 33% 1 0% 10 22% Virtually all students were within 1 grade of prediction, with a pleasing reduction in the proportion of students under-performing compared to last year. Higher level showed a slight under-performance (3 grade points in total). Standard level students performed significantly better than prediction (10 points in total). This reflects the guidance followed by several students early in the course to change to standard level. 1 student who scored 2 grades above prediction arrived late in school and during the IB1 year was catching up. During IB2 he showed improvement, but his final grade 7 was well beyond expectations, which is excellent for him. 6 IGCSE Grades Predicted Actual % G F E D C B A A* A*-C Comparison to Predictions Correct prediction Actual +1 Actual +2 Actual -1 Old:Overall, students achieved a Physics grade approx 0.4 of a grade higher than their average for all their other subjects. This was the first year of examination for the Edexcel syllabus. 83% of students achieved or exceeded their predictions. This syllabus was chosen for its more relevant and up-to-date content and structure. 7 8 Biology IB EXAM RESULT ANALYSIS May 2009 exam This was generally a highly motivated group of students with 81% achieving Band 5 or above, and 63% achieving Band 6 or above. Again the mean score from this group was well above the World Average. These are excellent results and reflective of the excellent rapport Wendy Carson had with the group. BIOLOGY HL Grade 7 6 2009 1 9 2008 3 2007 2 5 4 1 5 4 1 1 2 3 2 2 2 1 mean 5.35 5.4 4.4 GWA 4.18 4.1 Of predicted grades only one student achieved Grade 7 from the 8 predicted. This will be an area of investigation, to identify if this was due to across the board weaknesses in a particular area, or in the quality of written responses. While the prediction of so many Band 7s may have been optimistic, it surely spurred on the group to achieve to the best of their ability. BIOLOGY SL Grade 7 6 2009 1 1 2008 2007 - 5 2 4 3 4 6 6 5 3 2 3 1 2 - mean 4.42 4.1 4.2 GWA 4.21 4.3 Students again performed very well in SL in 2009, with all students either achieving their predicted grades or improving on them. Of particular note is the Grade 7 achieved by Manal Al-Adawi. Ray Zinzli did a fantastic job with these students with 83% achieving Band 4 or above. In 2010 the emphasis, for all IB students, will again be on the structure and quality of written responses incorporating the marking criteria. This will start a lot earlier with the aim of giving students the confidence to answer written response questions in terms of the different “command” terms used in each question. 9 IB EXAM RESULT ANALYSIS Chemistry CHEMISTRY HL Grade 7 6 5 2010 4 2 2009 6 6 2008 1 7 5 2007 5 5 2007 Global Average ie for the world 4 6 8 10 9 May 2010 Exam 3 8 1 2 5 2 1 mean 4.1 4.9 4.8 4.3 4.5 2010 Actual grades vs Predicted Grades: 14 ‘spot-on’ 3 were predicted 1 grade higher 2 were predicted 1 grade lower 1 was predicted 2 grades higher Comment: A disappointingly low Grade Point Average, although individual results largely matched predictions: 7 of the 8 Grade 3s were predicted. CHEMISTRY SL Grade 7 6 5 2010 3 2009 1 3 2008 1 2 2007 2 1 2007 Global Average ie for the world 4 3 3 3 5 3 3 2 3 2 2 1 5 1 2 mean 3.8 3.5 4.2 3.9 3.9 2010 Actual vs Predicted Grades: 5 spot on, 3 were predicted one grade higher 2 were predicted 1 grade lower Comment: Higher grade point average, but results pretty much as predicted. 10 2010 COMPONENT BREAKDOWN (Grades ranked in order) HL CHEMISTRY- 20 students Grades 7 6 Paper 1 m/choice 1 1 Paper 2 Structured 3 Paper 3 Option Practical Work 6 3 SL CHEMISTRY- 10 students Grades 7 6 5 4 P1 1 1 2 2 P2 4 P3 2 3 PW 1 1 4 3 5 4 2 4 5 3 4 3 2 1 4 6 6 2 6 2 2 1 3 7 3 6 - 1 1 2 2 1 5 5 - 1 1 3 Means 4.00 3.60 3.00 5.45 Means 4.3 2.5 4.0 4.8 2010 Comments At Higher Level the paper 3 options paper proved to the weakest. For the options chosen I considered this year's paper to be the most difficult of those set in recent years. The choice of options clearly needs to be reviewed: those students scoring very poorly did the Further Organic Chemistry’ option. This obviously proved to be more challenging unlike previous years. We intend to review our policy of choice. Another feature here is the grade point average for the Internal Coursework component. The means for both Higher and Standard Levels are above last years'. Whilst it is pleasing that the students did very well in this component, it does underline the total subjectivity of the external moderation procedure: Exactly the same Practical Scheme of Work as last year. Students were moderated down by between 3 and 5 marks (out of 48) this year. Last year students were moderated down between 13!! and 4 marks. I repeat: The moderation procedure is inconsistent and incomprehensible. 11 2009 COMPONENT BREAKDOWN (Grades ranked in order) HL CHEMISTRY- 22 students Grades 7 6 Paper 1 m/choice 2 8 Paper 2 Structured 3 7 Paper 3 Option 1 Practical Work 6 SL CHEMISTRY- 14 students Grades 7 6 5 4 P1 1 3 2 P2 1 2 2 3 P3 1 3 PW 4 8 5 4 7 2 12 3 6 1 2 4 8 3 5 3 2 2 5 2 - 3 2 5 - 1 1 7 - 2 8 - Means 5.18 5.27 3.05 4.91 Means 3.64 3.64 2.07 4.14 2009 Comments At Higher Level the multiple choice paper and the structured paper had significantly higher average than last years’. The averages for the Higher and Standard Level options paper were significantly lower than last years’ for the same options chosen. These were the most difficult papers as reflected in the grade boundaries: 26/40 for a grade 7 at standard level, 36/50 for a grade 7 at higher level. Perhaps we need to look closely at other option topics to ascertain their suitability for our students. Again the Practical Work moderation procedure remains a mystery. Whilst the grade point averages are good at Higher Level and satisfactory at Standard Level, they are significantly lower than last years’, with students being moderated down between 11 and 5 marks! (Similar to 2 years ago, but totally different from last year, for the same practical scheme of work and the same assessors!). None of the current Science staff have been trained in the new practical assessment / grading procedure. Perhaps this needs to be addressed. 12 IGCSE Chemistry Exam Performance Analysis May 2010 Comparison of 2008 with 2007 ( Numbers taking Core in Parentheses). For Core C is the top grade. A* A B C D E 2010 3 7 14 2009 4 11 13 12 9 (+2) 2 0 0 61 (+1) 1+(5) 1+(2) (1) 62 78.68% 10 12 (+3) 6 (+4) 3(+6) 87.27% 2008 3 89.36% 69.35% 2007 8 11 10 16 (+2) 19 (+1) 17 (+1) 4(+5) 4 (+6) 0+(7) 0 89.92% 70.40% ‘Spot on’ predictions Predicted 1 grade higher Predicted 1 grade lower Predicted 2 grades higher Predicted 2 grades lower F G Total A*-C Excluding Core 68 65.57% 54 A*-C Including Core 61.76% 29 19 11 8 1 Comments: Whilst 87% of predictions were spot on, or within 1 grade a feature of note is that 8 students were predicted 2 grades higher than their actual grades. In total 47% of students were over predicted. Whilst the significant decline in A*-C grades is a concern, it is totally in line with the overall School performance, indicative of a weak cohort. Predicted grades are based almost entirely on the mock examination result which is a composite of the 3 papers. A move forward would be to set all three papers as a mock, perhaps to achieve more accurate predictions. 13 Examination results analysis – Physics 2010 IB Grades Higher Standard Total 2010% 2009 % 2008 2008 % 7 0 0 2 0 4 0 0 6 1 4 5 15 9 6 18 5 2 2 4 12 22 4 12 4 7 5 12 36 35 9 27 3 3 9 12 36 28 11 33 2 0 0 0 0 2 3 9 1 0 0 0 0 0 0 0 Higher Level Grade Average was 4.08 compared to 4.86 in 2009 Standard Level Grade Average was 4.05 compared to 3.91 in 2009 Average IGCSE Physics score in 2007, (using A* = 8 points, A=7 etc) = 5.38 Average IGCSE Physics score in 2008, (using A* = 8 points, A=7 etc) = 5.80 (Therefore the group with the higher average IGCSE score has performed less well!) Component Analysis Higher Level 7 0 0 0 7 6 0 1 1 4 5 2 0 0 2 4 1 3 4 0 3 8 5 5 0 2 2 4 3 0 1 0 0 0 0 Standard Level 7 P1 1 P2 0 P3 0 Practical 3 6 2 2 1 7 5 3 3 3 7 4 5 3 4 3 3 7 4 8 0 2 2 6 2 0 1 0 2 2 0 P1 P2 P3 Practical Comparison to Predictions standard higher Correct prediction 10 0 Actual +1 Actual +2 Actual -1 Actual -2 Actual -3 3 0 0 0 5 9 2 3 0 1 14 Observations The standard level results were close to the expected ones and showed a slight increase on last year’s cohort. The higher level results were all, without exception, below the grades predicted and showed a considerable fall on the previous year’s. There is no evidence to suggest that this cohort was weaker than the previous year, as shown by the average IGCSE scores. All HL candidates obtained a B or higher at Physics IGCSE ( no data for Khadija Al Nabhani or Saja Al Balushi) Thankfully, the coursework marks were very good indeed, continuing the high standard of the previous year. Conclusions and Recommendations The HL results, in particular, were hugely disappointing. One of the key factors will be certainly have been the lack of time to complete the course. Despite bringing students in at the weekends and offering after school support on Tuesdays it was impossible to complete the course in a satisfactory way. The IB suggested time would appear to be a bare minimum. For in depth coverage and plenty of exam practice more time is needed. In practice, at the Sultan’s School, we tend to lose time due to weather, road closures and events beyond the school’s control; last year we also lost time to a variety of school initiatives (eg citizenship!) Recommend: Administration to prioritise teacher/student contact time Reintroduce CAT tests, or similar, to provide reliable bench marks to aid monitoring of exam performance School to look at how student independent learning can be improved across the curriculum 15 Analysis of Physics IGCSE Grades2010 G F E D C B A A* A*-C Predicted 0 0 0 3 12 14 20 0 46 Actual 0 0 2 5 7 13 18 4 42 % 0 0 4 10 14 27 37 8 86 Predicted 0 0 0 4 6 10 22 5 43 Actual 0 0 2 2 12 16 20 5 53 % 3 3 21 29 36 9 93 Predicted 0 1 3 8 12 16 12 3 43 Actual 0 0 2 5 14 18 11 4 47 % 0 0 4 9 26 33 20 7 87 2009 G F E D C B A A* A*-C 2008 G F E D C B A A* A*-C 16 Comparison to Predictions 2010 2009 2008 Correct prediction 28 35 28 Actual +1 Actual +2 Actual -1 Actual -2 1 5 8 7 9 2 3 11 11 12 Comments The results were very close to the expected ones. They show a small drop on last year’s excellent results. It would be nice to see more A*. From a personal point of view a number of very good physicists were at times held back by their average language skills. The slight increase in D grades causes some concern. Two students were 2 grades below the predicted: Aluzd Al Busaidi : B to D : this was a clerical error. An estimate of a C grade had been intended. Ahmed Al Maawaly B to D : this was a disappointment, and did not reflect his true ability, but may have been due to personal circumstances at the time. Alan Jones 24/8/2010 17 Biology EXAM RESULT ANALYSIS May 2010 exam This group spent most of the year playing ‘catch up’. They also had no internal assessments so it was necessary for them to come into school during the weekends. BIOLOGY HL Grade 6 5 2010 2 1 2008 3 1 2007 2 5 2007 Global Average ie for the world 4 5 1 2 3 2 2 2 mean 4.3 5.4 4.4 4.1 1 2010 Actual grades vs Predicted Grades: 3 ‘spot-on’, the rest attained one grade lower than their mock grade, which was used as their predicted grade. The mock examination was stopped because of rain, and students were marked on the work they had completed. I believe that the First Choice essay of most students contained the most familiar material, as a result, their mock marks gave them a more optimistic view of their knowledge and understanding of the material. The SL group spent teaching time hurriedly completing their assessments and students completed their options by independent study. BIOLOGY SL Grade 6 5 2010 1 3 2008 4 2007 3 2007 Global Average ie for the world 4 5 6 5 3 5 3 1 2 - mean 3.7 4.1 4.2 4.3 2010 Actual vs Predicted Grades: 2 spot on, 7 were predicted two grades higher. COMPONENT BREAKDOWN (Grades ranked in order) HL BIOLOGY- 10 students Paper 1 m/choice 7 6 Paper 2 Bulk 6 5 Paper 3 Option 6 6 Practical Work 6 6 6 5 6 6 5 4 5 5 4 3 3 5 3 3 3 5 3 3 3 5 3 3 3 5 3 3 3 4 3 2 2 4 18 IGCSE Biology 2010 2009 2008 2007 Exam Performance Analysis May 2010 A* A B C D E F G Total 1 15 12 8 4 4 9 10 8 15 10 5 (7) 10 (3) 6 9 1 (4) 3 (2) 8 7 (4) (3) 5 4 (3) (2) 4 1 59 54 52 48 10 13 A*-C Including Core 59 74 76 79 Comments Thanks to Peter Ross and Ray Zinsli for kindly tracking down past examination results for me. We shall continue with the good practice of including the Biology IGCSE Result analysis in the Science Department Handbook. I did not teach any Year 11 students last year, neither can I find any record of any students that may have been ear marked for being entered for ‘core only’ papers. There has clearly been a decline in the % A –C over the past years. This has been discussed at length at Middle Management meetings. However, although the data shows the increasing tail and spread of grades, it also highlights the number of Top grades, which have also been increasing over the years, as has the popularity of Biology as an IGCSE subject. In some cases, there were very large discrepancies between estimated grades and the final grades that were achieved. Students may have lessened their application to work after the mock examination. We need to find ways to maintain their motivation after the mocks so they do not just ‘switch off’. Revision sessions were offered but the uptake was minimal. It might be useful for the Head of Year 11 to publish a programme of support lessons after the Mocks, so that parents can see what is available. I would recommend that some students are not entered for the IGCSE if they are not making extra efforts at this time. Perhaps for certain students, attending extra support lessons may be the only way that they are able to be entered. August 2010 19 IB EXAM RESULT ANALYSIS Chemistry CHEMISTRY HL Grade 7 6 5 2011 4 3 2010 4 2 2009 6 6 2008 1 7 5 2007 5 5 2011 Global Average ie for the world 4 3 6 8 10 9 May 2011 Exam 3 2 8 1 2 5 2 1 1 mean 4.5 4.1 4.9 4.8 4.3 4.5 2011 Actual grades vs Predicted Grades: 8 ‘spot-on’ 1 was predicted 1 grade higher than achieved 2 were predicted 1 grade lower than achieved 1 was predicted 2 grades higher than achieved 1 was predicted 2 grades lower than achieved Comment: Grade Point Average significantly higher than last year. Two of the three grades 3 and 2 were advised not to take Higher Level Chemistry. Ahlam was predicted a Grade 5 and achieved 3, she did precisely the same in Physics: The predicted grades were based purely on the Mock Examinations, in both subjects Ahlam was very inconsistent during the year, but ‘turned it on’ for the mocks; obviously not the case for the Final Exam. CHEMISTRY SL Grade 7 6 5 2011 1 3 2010 3 2009 1 3 2008 1 2 2007 2 1 2011 Global Average ie for the world 4 9 3 3 3 5 3 7 3 2 3 2 2 3 1 5 1 2 mean 3.7 3.8 3.5 4.2 3.9 3.9 2011 Actual vs Predicted Grades: 12 spot on, 8 were predicted one grade higher than achieved 2 were predicted 1 grade lower than achieved 1 was predicted 2 grades lower than achieved Comment: Very similar grade point average, but results much as predicted. 20 2011 COMPONENT BREAKDOWN (Grades ranked in order) HL CHEMISTRY- 20 students Grades 7 6 Paper 1 m/choice 1 2 Paper 2 Structured 2 3 Paper 3 Option 1 2 Practical Work 0 0 5 6 5 2 6 SL CHEMISTRY- 10 students Grades 7 6 5 4 P1 0 1 1 9 P2 0 2 1 9 P3 0 3 2 5 PW 0 0 7 14 3 10 4 4 2 4 1 1 0 6 2 2 5 7 0 3 3 0 2 1 1 0 2 2 0 2 0 2 4 0 1 0 0 2 0 Means 4.31 5.00 3.46 4.38 Means 3.52 3.35 3.30 4.20 2011 Comments At Higher Level the paper 3 options paper again proved to the weakest (although better than last year). For the options chosen I considered this year's paper to be one of the most difficult of those set in recent years. Perhaps the choice of options and their delivery needs to be reviewed: Industrial Chemistry is largely self-studied due to time constraints. Another feature here is the grade point average for the Internal Coursework component. The means for both Higher and Standard Levels are below last years', again underlining the total subjectivity of the external moderation procedure: Exactly the same Practical Scheme of Work as last year. Students were moderated down between 6 and 15 marks! Last year, students were moderated down by between 1 and 5 marks (out of 48) this year. I repeat: The moderation procedure is inconsistent and incomprehensible. 21 IGCSE Chemistry Exam Performance Analysis A* 2011 7 A 6 B 16 C 16 D 11 E 9 2010 3 7 14 11 13 2008 3 10 12 12 (+3) 6 (+4) 3(+6) 9 68 (+2) 2 0 0 61 (+1) 1+(5) 1+(2) (1) 62 61.76% 2009 4 2007 8 11 10 16 (+2) 19 (+1) 17 (+1) 4(+5) 4 (+6) 0+(7) 0 70.40% ‘Spot on’ predictions Predicted 1 grade higher Predicted 1 grade lower Predicted 2 grades higher Predicted 2 grades lower F 4 G 3 May 2011 Total A*-C 70 64.5% 54 78.68% 69.35% 30 12 (Most were predicted D’s or E’s) 15 (Most were predicted C’s or B’s) 10 (Again most predicted D’s or E’s) 3 Comments: An increase in the A*-C percentage, with a significant increase in A* grades. However, measures are planned to attempt to reduce the number of D grades, which again is disappointing. 22 Biology IB EXAM RESULT ANALYSIS BIOLOGY HL Grade 7 6 5 4 2011 1 5 5 2 2010 2 1 5 2008 3 1 1 2007 2 5 2 2007 Global Average ie for the world May 2011exam 3 2 2 2 1 mean 5.4 4.3 5.4 4.4 4.1 7 ‘spot-on’, 2 were predicted 1 grade higher than achieved 3 were predicted 1 grade lower than achieved 1 was predicted 2 grades lower than achieved 2011 Actual grades vs Predicted Grades: Comment BIOLOGY SL Grade 6 5 2011 2 2010 1 3 2008 4 2007 3 2007 Global Average ie for the world 2011 Actual vs Predicted Grades: 4 1 5 6 5 3 3 5 3 1 2 2 - mean 3.4 3.7 4.1 4.2 4.3 2 spot on, 1 was predicted one grade higher than achieved 3 were predicted 1 grade lower than achieved 1 was predicted 2 grades higher than achieved 1 was predicted 2 grades higher than achieved Comment 23 COMPONENT BREAKDOWN (Grades ranked in order) HL BIOLOGY- 10 students Paper 1 m/choice 7 6 Paper 2 Bulk 6 5 Paper 3 Option 6 6 Practical Work 6 6 SL BIOLOGY- 15 students P1 6 6 5 5 P2 6 6 6 6 P3 6 6 6 4 PW 4 4 4 4 6 5 6 6 4 4 4 4 4 4 4 4 5 4 5 5 4 4 4 4 4 3 3 5 4 4 4 4 3 3 3 5 3 4 4 3 3 3 3 5 3 3 4 3 3 3 3 3 3 3 3 5 3 3 3 3 3 3 3 4 3 2 3 3 3 2 2 4 3 2 3 3 2 1 2 3 Comments The Practical Work moderation remains something of a mystery, but there was most definitely a pattern of optimistic grading in SL this year, which may have influenced the optimistic predictions for the final grades (+2 grade) overall. I am pleased with the HL practical work scores. Next year the assessments will be marked and moderated within the department throughout the year. HL- Only Sara Ferwati achieved a Grade 7 in Paper 1 SL- A good collection of Grade 6 in all three papers. Conclusion: The pattern of the IB results is similar to the expected results. It is pleasing to note that our HL average is higher than the world average this year. We also need to appreciate that the ability range of students taking IB is widening. We are pleased to note that Adnan has been able to place students with IB Certificates and hope that future Year 12 students are given a realistic picture of their capabilities, so that they all can succeed at their appropriate level. August 2011 24 Examination results analysis – Physics 2011 IB Grades Higher Standard Total 2011% 2010% 2009 % 2008 2008 % 7 0 0 0 0 0 4 0 0 6 0 0 0 0 15 9 6 18 5 6 1 7 20 12 22 4 12 4 10 4 14 40 36 35 9 27 3 6 8 14 40 36 28 11 33 2 0 0 0 0 0 2 3 9 1 0 0 0 0 0 0 0 0 Higher Level Grade Average was 4.00 compared to 4.08 in 2010 Standard Level Grade Average was 3.46 compared to 4.05 in 2010 Average IGCSE Physics score in 2007, (using A* = 8 points, A=7 etc) = 5.38 Average IGCSE Physics score in 2008, (using A* = 8 points, A=7 etc) = 5.80 (Therefore the group with the higher average IGCSE score has performed less well!) Component Analysis Higher Level 7 0 0 0 7 6 0 1 1 4 5 2 0 0 2 4 1 3 4 0 3 8 5 5 0 2 2 4 3 0 1 0 0 0 0 Standard Level 7 P1 1 P2 0 P3 0 Practical 3 6 2 2 1 7 5 3 3 3 7 4 5 3 4 3 3 7 4 8 0 2 2 6 2 0 1 0 2 2 0 P1 P2 P3 Practical 25 Comparison to Predictions standard higher Correct prediction 10 0 Actual +1 Actual +2 Actual -1 Actual -2 Actual -3 3 0 0 0 5 9 2 3 0 1 26 Observations The standard level results were close to the expected ones and showed a slight increase on last year’s cohort. The higher level results were all, without exception, below the grades predicted and showed a considerable fall on the previous year’s. There is no evidence to suggest that this cohort was weaker than the previous year, as shown by the average IGCSE scores. All HL candidates obtained a B or higher at Physics IGCSE ( no data for Khadija Al Nabhani or Saja Al Balushi) Thankfully, the coursework marks were very good indeed, continuing the high standard of the previous year. Conclusions and Recommendations The HL results, in particular, were hugely disappointing. One of the key factors will be certainly have been the lack of time to complete the course. Despite bringing students in at the weekends and offering after school support on Tuesdays it was impossible to complete the course in a satisfactory way. The IB suggested time would appear to be a bare minimum. For in depth coverage and plenty of exam practice more time is needed. In practice, at the Sultan’s School, we tend to lose time due to weather, road closures and events beyond the school’s control; last year we also lost time to a variety of school initiatives (eg citizenship!) Recommend: Administration to prioritise teacher/student contact time Reintroduce CAT tests, or similar, to provide reliable bench marks to aid monitoring of exam performance School to look at how student independent learning can be improved across the curriculum Practical Equipment List There are detailed inventories available in the intranet, path: ‘Science Department Shared Resources’ ‘Lab Technicians’ 27