BEHAVIOR OF GASES CHAPTER 12
Standard Temperature and Pressure
• Standard temperature and pressure is given the symbol STP.
– It is a reference point for some gas calculations.
• Standard P 1.00000 atm or 101.3 kPa
• Standard T 273.15 K or 0.00
o C
– Gas laws must use the Kelvin scale to be correct.
• Relationship between Kelvin and centigrade.
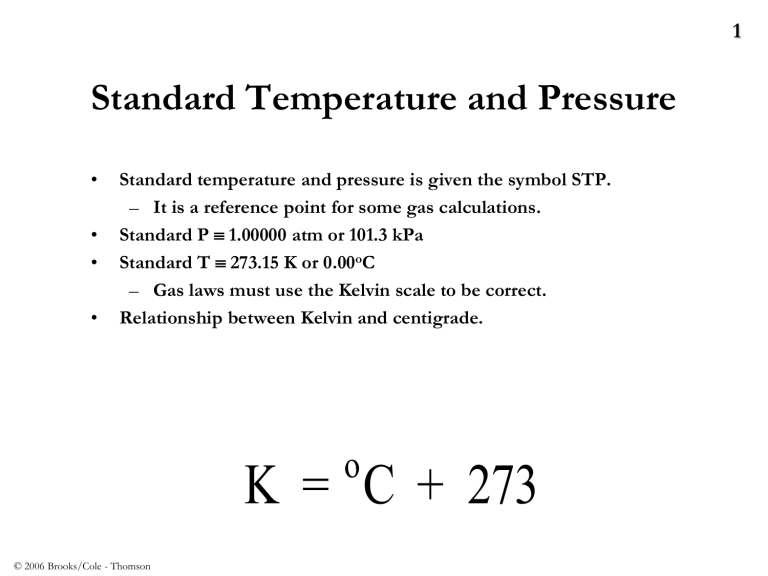
1 o
K = C + 273
© 2006 Brooks/Cole - Thomson
Boyle’s Law:
The Volume-Pressure Relationship
• V 1/P or
• V= k (1/P) or PV = k
• P
1
V
1
= k
1 for one sample of a gas.
• P
2
V
2
= k
2 for a second sample of a gas.
• k
1
= k
2 for the same sample of a gas at the same T.
• Thus we can write Boyle’s Law mathematically as P
1
V
1
= P
2
V
2
Robert Boyle (1627-1691).
Son of Earl of Cork,
Ireland.
2
© 2006 Brooks/Cole - Thomson
Charles’ Law:
The Volume-Temperature Relationship;
The Absolute Temperature Scale
• Mathematical form of Charles’ law.
V
1
T
1
V
T or V = kT or
V
T
k
k and
V
2
T
2
k however th e k' s are equal so
V
1
T
1
V
2 in the
T
2 most useful form
Jacques Charles (1746-1823).
Isolated boron and studied gases. Balloonist.
© 2006 Brooks/Cole - Thomson
3
Charles’ Law:
The Volume-Temperature Relationship;
The Absolute Temperature Scale
35
30
25
10
5
20
15
Gases liquefy before reaching 0K
0
0 50 100 150 200 250 300 350 400
Temperature (K)
© 2006 Brooks/Cole - Thomson absolute zero = -273.15 0 C
4
The Combined Gas Law Equation
• Boyle’s and Charles’ Laws combined into one statement is called the combined gas law equation.
– Useful when the V, T, and P of a gas are changing.
Boyle' s Law Charles' Law
P
1
V
1
P
2
V
2
V
1
T
1
V
2
T
2
For a given sample of gas : The combined gas law is :
P V
T
k
P
1
V
1
T
1
P
2
V
2
T
2
5
© 2006 Brooks/Cole - Thomson
Avogadro’s Law and the
Standard Molar Volume
• Avogadro’s Law states that at the same temperature and pressure, equal volumes of two gases contain the same number of molecules (or moles) of gas.
• If we set the temperature and pressure for any gas to be STP, then one mole of that gas has a volume
called the standard molar volume.
• The standard molar volume is 22.4 L at STP.
– This is another way to measure moles.
– For gases, the volume is proportional to the number of moles.
• 11.2 L of a gas at STP = 0.500 mole
– 44.8 L = ? moles
© 2006 Brooks/Cole - Thomson
6
Boyle’s Law:
The Volume-Pressure Relationship
• At 25 o C a sample of He has a volume of 4.00 x 10 2 mL under a pressure of 7.60 x 10 2 torr. What volume would it occupy under a pressure of 2.00 atm at the same T?
7
© 2006 Brooks/Cole - Thomson
Charles’ Law:
The Volume-Temperature Relationship;
The Absolute Temperature Scale
• A sample of hydrogen, H the same pressure?
2
, occupies 1.00 x 10 2 mL at 25.0
o C and 1.00 atm. What volume would it occupy at 50.0
o C under
8
© 2006 Brooks/Cole - Thomson
The Combined Gas Law Equation
• A sample of nitrogen gas, N would it occupy at STP?
2
, occupies 7.50 x 10 2 mL at
75.0
0 C under a pressure of 8.10 x 10 2 torr. What volume
9
© 2006 Brooks/Cole - Thomson
Avogadro’s Law and the
Standard Molar Volume
• One mole of a gas occupies 36.5 L and its density is 1.36 g/L at a given temperature and pressure. (a) What is its molar mass? (b) What is its density at STP?
10
© 2006 Brooks/Cole - Thomson
Summary of Gas Laws:
The Ideal Gas Law
• Boyle’s Law - V 1/P (at constant T & n)
• Charles’ Law – V T (at constant P & n)
• Avogadro’s Law – V n (at constant T & P)
• Combine these three laws into one statement
V nT/P
• Convert the proportionality into an equality.
V = nRT/P
• This provides the Ideal Gas Law.
PV = nRT
• R is a proportionality constant called the universal gas constant. 0.08206 L atm mol -1 K -1
11
© 2006 Brooks/Cole - Thomson
Summary of Gas Laws:
The Ideal Gas Law
• What volume would 50.0 g of ethane, C
2
H
6
, occupy at 1.40 x 10 2 o C under a pressure of 1.82 x 10 3 torr?
12
© 2006 Brooks/Cole - Thomson
Summary of Gas Laws:
The Ideal Gas Law
• Calculate the number of moles in, and the mass of, an 8.96 L sample of methane, CH
4
, measured at standard conditions?
13
© 2006 Brooks/Cole - Thomson
14
Dalton’s Law of Partial Pressures
• Dalton’s law states that the pressure exerted by a mixture of gases is the sum of the partial pressures of the individual gases.
P total
= P
A
+ P
B
+ P
C
+ .....
•Vapor Pressure is the pressure exerted by a substance’s vapor over the substance’s liquid at equilibrium.
John Dalton
1766-1844
© 2006 Brooks/Cole - Thomson
Dalton’s Law of Partial Pressures
• If 1.00 x 10 2 mL of hydrogen, measured at 25.0 o C and 3.00 atm pressure, and 1.00 x 10 2 mL of oxygen, measured at 25.0 o C and 2.00 atm pressure, were forced into one of the containers at 25.0 o C, what would be the pressure of the mixture of gases?
15
© 2006 Brooks/Cole - Thomson
Dalton’s Law of Partial Pressures
16
• A sample of hydrogen was collected by displacement of water at 25.0 o C. The atmospheric pressure was 748 torr. What pressure would the dry hydrogen exert in the same container?
© 2006 Brooks/Cole - Thomson
Mass-Volume Relationships in Reactions Involving Gases
•In this section we are looking at reaction stoichiometry, like in
Chapter 3, just including gases in the calculations.
2 KClO
3 (s)
2
&
2 KCl
(s)
+ 3 O
2 ( g)
2 mol KClO
3 yields 2 mol KCl and 3 mol O
2(122.6g) yields 2 (74.6g) and 3 (32.0g)
2
Those 3 moles of O
2 can also be thought of as:
3(22.4L) or
67.2 L at STP
17
© 2006 Brooks/Cole - Thomson
Mass-Volume Relationships in
Reactions Involving Gases
• What volume of oxygen measured at STP, can be produced by the thermal decomposition of 120.0 g of KClO
3
?
18
© 2006 Brooks/Cole - Thomson
Real Gases:
Deviations from Ideality
• Real gases behave ideally at ordinary temperatures and pressures.
• At low temperatures and high pressures real gases do not behave ideally.
• The reasons for the deviations from ideality are:
1.
The molecules are very close to one another, thus their volume is important.
2.
The molecular interactions also become important.
19
J. van der Waals, 1837-1923,
Professor of Physics,
Amsterdam. Nobel Prize 1910.
© 2006 Brooks/Cole - Thomson
Real Gases:
Deviations from Ideality
• van der Waals’ equation accounts for the behavior of real gases at low temperatures and high pressures.
P +
2 n a
V
2
V
nb
nRT
• The van der Waals constants a and b take into account two things:
1.
a accounts for intermolecular attraction a.
For nonpolar gases the attractive forces are London Forces b.
For polar gases the attractive forces are dipole-dipole attractions or hydrogen bonds.
2. b accounts for volume of gas molecules
At large volumes a and b are relatively small and van der Waal’s equation reduces to ideal gas law at high temperatures and low pressures.
© 2006 Brooks/Cole - Thomson
20
Real Gases:
Deviations from Ideality
• Calculate the pressure exerted by 84.0 g of ammonia, NH
3
5.00 L container at 200. o C using the ideal gas law.
, in a
21
PV = nRT
P = nRT/V n = 84.0g * 1mol/17 g T = 200 + 273
P = (4.94mol)(0.08206
L atm mol -1 K -1
)(473 K)
(5 L)
P = 38.3 atm
© 2006 Brooks/Cole - Thomson
Real Gases: Deviations from Ideality
• Calculate the pressure exerted by 84.0 g of ammonia, NH container at 200. o C using the van der Waal’s equation. The van der Waal's constants for ammonia are: a = 4.17 atm L 2 mol -2
3
, in a 5.00 L b = 3.71x10
-2 L mol -1
P +
2 n a
V
2
V
nb
nRT
P
nRT
V nb
2
n
V
2 a n = 84.0g * 1mol/17 g T = 200 + 273
P =
(4.94mol)(0.08206 L atm mol -1 K -1 )(473K) (4.94 mol) 2 *4.17 atm L 2 mol -2
5 L – (4.94 mol*3.71
E
-2 L mol -1 ) (5 L) 2
P = 39.81 atm – 4.07 atm = 35.74
P = 38.3 atm
7% error
22
© 2006 Brooks/Cole - Thomson