Membrane Structure - Madison Public Schools
advertisement

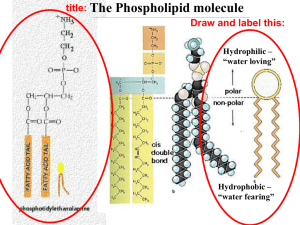
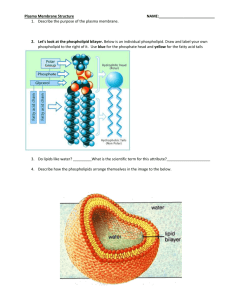
The Structure of the Cell Membrane • http://www.youtube.com/watch?v=ULR79TiUj 80&feature=related The Structure of Membrane Lipids Membrane-forming lipids contain both a polar, hydrophilic region and a nonpolar, hydrophobic region. • Phospholipids are amphipathic: – The “head” region, consisting of a glycerol, a phosphate, and a charged group, contains highly polar covalent bonds. – The “tail” region is comprised of two nonpolar fatty acid • When placed in solution, the phospholipid heads interact with water while the tails do not, allowing these lipids to form membranes. Phospholipid Bilayers • Phospholipid bilayers form when two sheets of phospholipid molecules align. The hydrophilic heads in each layer face a surrounding solution, while the hydrophobic tails face one another inside the bilayer. • Phospholipid bilayers form spontaneously, with no outside input of energy required. Phospholipids and Water • Phospholipids do not dissolve when they are placed in water. • Water molecules interact with the hydrophilic heads but not with the hydrophobic tails. – This drives the hydrophobic tails together. • Upon contact with water phospholipids form either: – Micelles • Heads face the water and tails face each other. – Phospholipid bilayers (lipid bilayers) Selective Permeability of Lipid Bilayers • The permeability of a structure is its tendency to allow a given substance to pass across it. Phospholipid bilayers have selective permeability. – Small or nonpolar molecules move across phospholipid bilayers quickly. – Charged or large polar substances cross slowly, if at all. Many Factors Affect Membrane Permeability • Many factors influence the behavior of the membrane: – Number of double bonds between the carbons in the phospholipid’s hydrophobic tail – Length of the tail – Number of cholesterol molecules in the membrane – Temperature Bond Saturation and Membrane Permeability • Double bonds between carbons in a hydrocarbon chain can cause a “kink” in the hydrocarbon chain, preventing the close packing of hydrocarbon tails, and reducing hydrophobic interactions. – Unsaturated hydrocarbon chains have at least one double bond. – Hydrocarbon chains without double bonds are termed saturated. • Saturated fats have more chemical energy than unsaturated fats. • Membranes with unsaturated phospholipid tails are much more permeable than those formed by phospholipids with saturated tails. Other Factors That Affect Permeability • Hydrophobic interactions become stronger as saturated hydrocarbon tails increase in length. – Membranes containing phospholipids with longer tails have reduced permeability. • Adding cholesterol to membranes increases the density of the hydrophobic section. – Cholesterol decreases membrane permeability. • Membrane fluidity decreases with temperature because molecules in the bilayer move more slowly. – Decreased membrane fluidity causes decreased permeability. Cell Membrane Review Questions You want to construct a molecule that will migrate easily through a cell membrane. What properties should you give your molecule? a. It should be small and charged. b. It should be large and hydrophilic. c. It should be hydrophobic. d. It should be hydrophilic. Which of the following events would you expect to be spontaneous in aqueous (water) solutions? a. net movement of calcium ions from 1.5 molar CaCl2 to 2.0 molar CaCl2 b. net movement of sugar molecules from 0.5 molar sugar to 0.4 molar sugar c. net movement of water from a 1.2-molar solution of NaCl to a 0.9molar solution of NaCl d. net movement of water from 0.3 molar sugar to pure water Yeast cells require a protein to transport glucose from the environment into the cell. You would expect that protein to be _____. a. a transmembrane (integral) protein b. a peripheral membrane protein c. present only in the cytoplasm d. an ion channel What does it mean for the membrane to be selectively permeable?