Experimental results
advertisement
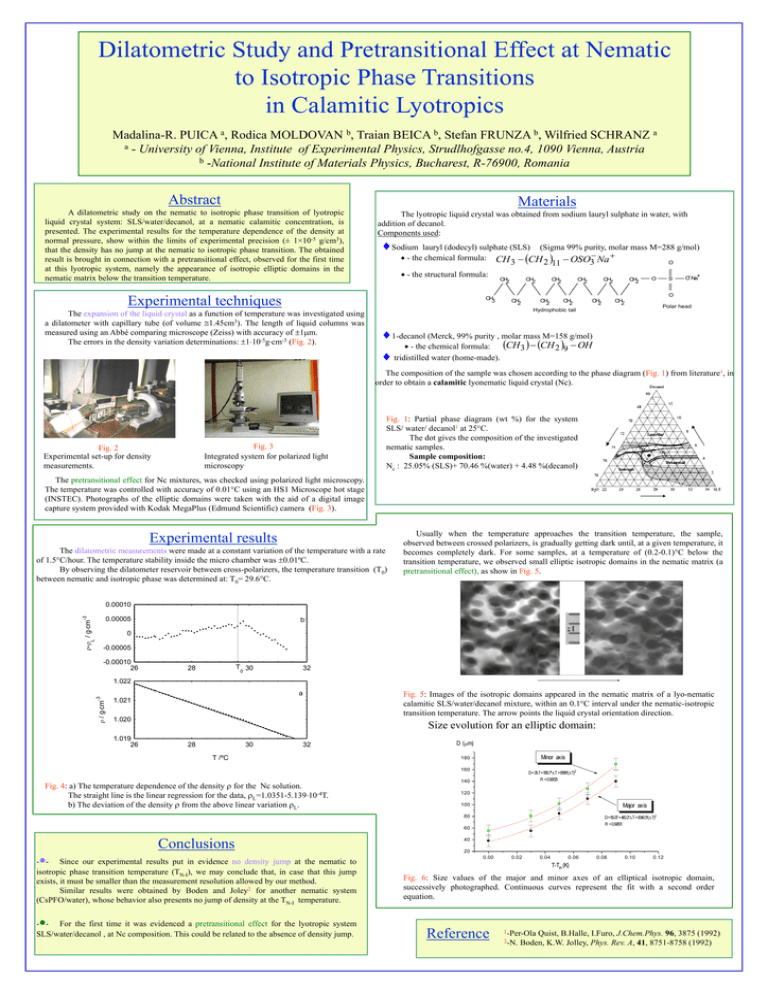
Dilatometric Study and Pretransitional Effect at Nematic to Isotropic Phase Transitions in Calamitic Lyotropics Madalina-R. PUICA a, Rodica MOLDOVAN b, Traian BEICA b, Stefan FRUNZA b, Wilfried SCHRANZ a a - University of Vienna, Institute of Experimental Physics, Strudlhofgasse no.4, 1090 Vienna, Austria b -National Institute of Materials Physics, Bucharest, R-76900, Romania Abstract Materials A dilatometric study on the nematic to isotropic phase transition of lyotropic liquid crystal system: SLS/water/decanol, at a nematic calamitic concentration, is presented. The experimental results for the temperature dependence of the density at normal pressure, show within the limits of experimental precision (± 1×10-5 g/cm3), that the density has no jump at the nematic to isotropic phase transition. The obtained result is brought in connection with a pretransitional effect, observed for the first time at this lyotropic system, namely the appearance of isotropic elliptic domains in the nematic matrix below the transition temperature. The lyotropic liquid crystal was obtained from sodium lauryl sulphate in water, with addition of decanol. Components used: Sodium lauryl (dodecyl) sulphate (SLS) (Sigma 99% purity, molar mass M=288 g/mol) - the chemical formula: CH CH OSO Na O 3 2 11 3 - the structural formula: Experimental techniques CH3 The expansion of the liquid crystal as a function of temperature was investigated using a dilatometer with capillary tube (of volume 1.45cm3). The length of liquid columns was measured using an Abbé comparing microscope (Zeiss) with accuracy of 1m. The errors in the density variation determinations: 110-5gcm-3 (Fig. 2). CH2 CH2 CH2 CH2 CH2 CH2 O S O- Na+ O CH2 CH2 CH2 Hydrophobic tail CH2 CH2 Polar head 1-decanol (Merck, 99% purity , molar mass M=158 g/mol) CH 3 CH 2 9 OH - the chemical formula: tridistilled water (home-made). The composition of the sample was chosen according to the phase diagram (Fig. 1) from literature1, in order to obtain a calamitic lyonematic liquid crystal (Nc). Fig. 3 Integrated system for polarized light microscopy Fig. 2 Experimental set-up for density measurements. Fig. 1: Partial phase diagram (wt %) for the system SLS/ water/ decanol1 at 25°C. The dot gives the composition of the investigated nematic samples. Sample composition: Nc : 25.05% (SLS)+ 70.46 %(water) + 4.48 %(decanol) The pretransitional effect for Nc mixtures, was checked using polarized light microscopy. The temperature was controlled with accuracy of 0.01°C using an HS1 Microscope hot stage (INSTEC). Photographs of the elliptic domains were taken with the aid of a digital image capture system provided with Kodak MegaPlus (Edmund Scientific) camera (Fig. 3). Experimental results The dilatometric measurements were made at a constant variation of the temperature with a rate of 1.5°C/hour. The temperature stability inside the micro chamber was 0.01ºC. By observing the dilatometer reservoir between cross-polarizers, the temperature transition (T0) between nematic and isotropic phase was determined at: T0= 29.6°C. Usually when the temperature approaches the transition temperature, the sample, observed between crossed polarizers, is gradually getting dark until, at a given temperature, it becomes completely dark. For some samples, at a temperature of (0.2-0.1)°C below the transition temperature, we observed small elliptic isotropic domains in the nematic matrix (a pretransitional effect), as show in Fig. 5. -3 0.00010 - / gcm 0.00005 b L 0 -0.00005 -0.00010 26 T 30 0 28 32 / gcm -3 1.022 a Fig. 5: Images of the isotropic domains appeared in the nematic matrix of a lyo-nematic calamitic SLS/water/decanol mixture, within an 0.1°C interval under the nematic-isotropic transition temperature. The arrow points the liquid crystal orientation direction. 1.021 1.020 1.019 26 Size evolution for an elliptic domain: 28 30 32 T /ºC D (m) Minor axis 180 160 Fig. 4: a) The temperature dependence of the density for the Nc solution. The straight line is the linear regression for the data, L=1.0351-5.13910-4T. b) The deviation of the density from the above linear variation L. D= 36.7 + 566.7* T + 6589*( T)2 R = 0.99725 140 120 Major axis 100 D= 56.07 + 480.2* T + 8340.5*( T)2 R = 0.99831 80 60 Conclusions - - Since our experimental results put in evidence no density jump at the nematic to isotropic phase transition temperature (TN-I), we may conclude that, in case that this jump exists, it must be smaller than the measurement resolution allowed by our method. Similar results were obtained by Boden and Joley2 for another nematic system (CsPFO/water), whose behavior also presents no jump of density at the TN-I temperature. - - For the first time it was evidenced a pretransitional effect for the lyotropic system SLS/water/decanol , at Nc composition. This could be related to the absence of density jump. 40 20 0.00 0.02 0.04 0.06 0.08 0.10 0.12 T-Tin (K) Fig. 6: Size values of the major and minor axes of an elliptical isotropic domain, successively photographed. Continuous curves represent the fit with a second order equation. Reference 1-Per-Ola Quist, B.Halle, I.Furo, J.Chem.Phys. 96, 3875 (1992) 2-N. Boden, K.W. Jolley, Phys. Rev. A, 41, 8751-8758 (1992)







