How electrons produce color
advertisement

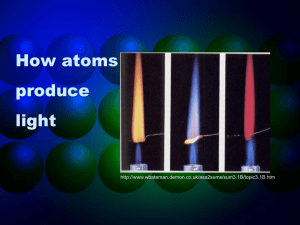
How atoms produce light http://www.wbateman.demon.co.uk/asa2sums/sum3.1B/topic3.1B.htm LIGHT • A generic term for electromagnetic radiation (like energy from the sun) • Small packets of energy that travel as waves…these packets are called PHOTONS • Wave properties for a given photon are all related to each other Wave properties • Since light is a form of energy, we know that it travels as a wave. Waves have a set of properties that are related to each other. • WAVE A WAVE B • Wavelength- The distance between two equal parts of a wave • Frequency – The # of waves that pass a point each second • Speed – How fast a wave travels (all light travels at the same speed) Questions: WAVE A WAVE B • Which of the two light samples above has a longer wavelength? • If both light samples are travelling by you at the same speed, which will have more waves pass by you in one minute? • From your answer to # 2, which light has the higher frequency? • Are wavelength and frequency related directly or inversely? Electromagnetic Spectrum • Which has longer waves, gamma rays or microwaves? • Which has shorter waves, orange light or green light? • Which has shorter waves, violet light or ultraviolet light? • Which has longer waves, red light or infrared light? • In the spectrum modeled above, which type of radiation do you think has the highest energy? • Based on your answers above, are energy and wavelength directly or inversely proportional? • Are energy and frequency directly or inversely proportional? • Which has higher energy, orange light or green light? • Which has higher energy, red light or infrared light? • Which has higher energy, violet light or ultraviolet light? Spectroscope • A simple spectroscope has a flat prism that separates light so we can see the individual colors. http://asd-www.larc.nasa.gov/edu_act/simple_spec.html “White Light” Pass out Spectroscopes and colored pecils • Aim the vertical slit toward the incandescent light source (the sun) • You will see the light through the slit. • Without moving the spectroscope, drift your eyes to the right until you see the numbers on the scale. • What do you see over the numbers? Continuous Spectrum= all colors There are no “blank spots” in the spectrum! http://physics.uoregon.edu/~jimbrau/astr122/Notes/Chapter3.html Why continuous spectrum? • A solid is heated…all of its atoms/molecules and their parts move really fast • Energy is given off as the atoms constantly vibrate. • Photons of all colors can be emitted. • (given off) • All colors blend into “white light” Another type of spectrum • Aim the vertical slit at the overhead lights in the room. • How does this look different from the incandescent light? • Do the colors that show up always show up in the same place? Brightline Spectrum When only certain photons are observed, it means that only light packets of a particular type are being emitted! • Each photon has a specific energy value… • …so only certain energy exchanges are happening within the heated substance. • …so there must only be certain ways of changing the energy in the substance! How? • This can be explained by the movement of electrons! • We know from the Bohr model that atoms have “layers” of electrons called energy levels. • Each energy level has electrons with a certain amount of energy in them that matches the level. • When the electrons change levels, they have to gain or lose energy to do so. • Each time they lose energy, they emit (release) a bundle of energy. • We see that bundle as a photon! Line Spectrums • When heated, the electrons in an atom are excited. Electrons “jump” to higher energy levels. • When they go back down they release energy (photons) as light. • This can be recorded as a line spectrum. Line Spectrums • Different elements emit different wavelengths of light because each element has a different electron arrangement. • Each element has a different pattern of wavelengths and a different line spectrum. Photons and Atoms • Photon is just the name for a small piece of light • Electron Transition – when an electron moves from one level to another – When an electron transitions to a higher energy level, a photon is absorbed. – When an electron transitions to a lower energy level, a photon is emitted. • Techniques like spectroscopy have allowed us to discover new elements like Caesium and Rubidium. • Helium was discovered by looking at the line spectrum of the sun during an eclipse! Conclusion • Atoms only emit photons of specific energies • That is why we can use atomic emissions spectra to identify elements in an unknown sample.