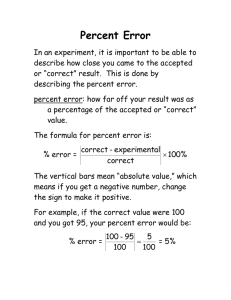
Fundamental Forces
advertisement

Experimental Methods and Physics Skills /Astrophysical Skills and Techniques Physics and Astrophysics Laboratory Tutors: Dr.A.Mahendrasingam (Singam) a.mahendrasingam@keele.ac.uk Dr.D.McLaughlin d.e.mclaughlin@keele.ac.uk Physics/Astrophysics Laboratory Lecture Course Provide an education in the knowledge required to become a Physicist/Astrophysicist Laboratory Skills Educating students in some of the skills required to be a Physicist/Astrophysicist Physics/Astrophysics Laboratory Module Structure: Two strands Practical abilities to perform experiments and the intellectual ability to analyse and access the results of experiments (strand 1) Basic computational skills (strand 2) Physics/Astrophysics Laboratory The experimental Methods Strand Expected to perform 8 laboratory experiments during the two semesters ( 5 in semester one, 3 in semester 2) Your laboratory diary will be marked at the end of each laboratory session. From this mark you will receive a final mark for each completed experiments Each of these 8 experiments should take you 2 weeks ( 2 x 3 hour laboratory sessions) Physics/Astrophysics Laboratory The Computing Strand Spreadsheets Programming Physics/Astrophysics Laboratory Assessment Semester one Strand 1 ( 90 marks) Strand 2 ( 10 marks) Semester two Strand 1 ( 90 marks) Strand 2 ( 10 marks) Physics/Astrophysics Laboratory Semester one Experimental Strand Bench work (Lab diary for 5 completed experiments) Mark 30 Report 1 Report 2 30 30 Computing Strand Spreadsheet exercises associated with any two Experiments 10 Total 100 Physics/Astrophysics Laboratory Requirement to pass the Laboratory 40 marks out of 100 available ( i.e. 40%) Submit 4 (2 reports/semester) satisfactory laboratory reports for strand 1 Laboratory contributes 20% to each lecture modules. Physics/Astrophysics Laboratory Semester 1 Laboratory – Contribution to the Lecture Modules PHY-10022 20% PHY-10024 20% Semester 2 Laboratory – Contribution to the Lecture Modules PHY-10020 20% PHY-10021/23 20% Laboratory Class (Thursday 14:00 – 17:00) Introduction to Laboratory Class – Friday (4/10/13) 10:00 – 11:00 in LJ 1.75 First lab session (Lecture on error and Data Analysis) – Thursday (10/10/13) 14:00 – 15:00 in CBA 0.061 First lab session (Worksheet on error and Data Analysis) – Thursday (10/10/13) 15:00 – 17:00 in LJ0.26 (Faculty Computing Lab) Experiment on (B) simple Harmonic Motion / Data Analysis and Processing 10/10/13 – 24/10/13 Week 1 Week 2 Week 3 Week 4 Experiments A and C - M Week 5 Maths test 2-3pm Maths test 2-3pm Lab 3-5pm Lab 3-5pm Week 6 Week 7 Week 8 Week 9 Week 10 Week 11 Report #1 Deadline 4.00 PM 22/11/13 Week 12 Physics/Astrophysics Laboratory Hardback notebook ( You should be able to buy the notebook from the Stores (LJ0.36 or Phil ) Your copy of the Lab. Manual Additional information about the laboratory can be found in the module pages for PHY-10022/PHY-10024 on VLE (http://students.keele.ac.uk) Laboratory Notebook – Section Front of your notebook should contain: Name: Address: School of Physical and Geographical Sciences Each new experiment should start on a new page Title of the experiment should be recorded at the start of the each new experiment. Date should be recorded at the beginning of each laboratory session Results should be recorded with a short sentence which is sufficiently explanatory that you or someone else can understand it Record all your data/measurements as you take them Laboratory Notebook Always record the units of your measurements along with the measurements themselves If your data is taken for a certain period of time or a certain number of oscillations etc. then always record this fact along with the measurements themselves. If you plot or fit your data using one of the computer programs, make a printout of the program output (usually a graph) and attach (glue, sellotape, staple) it into your notebook. If you use the spreadsheet to analyse the data, make a printout of the spreadsheet and attach (glue, sellotape, staple) it into your notebook. Laboratory Notebook If you decide that a set of measurements is incorrect for some reason don’t obliterate it in your notebook. Instead simply draw one diagonal line through it and make a note why you have discarded it. If at a later date you change your mind (or if a staff supervisor or post-graduate demonstrator persuades you to change your mind) you won’t have to re-take the data again. As long as it can still be read it can be used. Make a note of the pieces of apparatus that you are using in your experiment, e.g. radioactive source B, A.C. circuit box G, a Farnell oscilloscope serial number F831GBX etc. If for some reason a piece of your apparatus is removed (it shouldn’t be but !) then we can recover it if we know the number and you can continue your experiment without having to start again. At the end of your experiment you should summarise your results, tabulating clearly the values you have obtained for any derived quantities (and their error bars) with suitable notes explaining what each is. Further details can be found in section 2.1 of the laboratory manual. PC Lab (LJ1.27) You should able to logon to the computers in the PC Lab using your university computer userid and password. Also make sure that you save all your work on your network drive (S:). The networked laser printer in the PC Lab can be used to print your work in the laboratory. Initially you will be given a free 50 pages print quota. Additional print quota can be purchased from Phil. A key is required to gain access to PC lab. You can obtain a key from Phil by paying a refundable deposit. You can also use the PCs in the PC Lab in LJ0.026 (Faculty Computing Lab) using Keele Card. SAFETYBRIEFING FOR PHYSICS/ASTROPHYSICS STUDENTS Safety General safety policy Emergency procedures Dos and Don'ts General Safety Policy The School of Physical and Geographical Sciences must Provide safe experiments in a safe environment Establish emergency procedures Provide safety information and guidance It is your responsibility to Take reasonable care for your own health and safety Take reasonable care for others’ health and safety by complying with the safety rules in the Lennard-Jones Laboratories Emergency Procedures – FIRE ALARM THE FIRE ALARM IS A CONTINUOUSLY SOUNDING SIREN The entire building MUST be evacuated if the siren sounds Leave either through the foyer or go right along the corridor and leave through the rear building DO NOT PANIC DO NOT TAKE ANY PERSONAL RISKS DO NOT USE THE SERVICE LIFT if the fire alarm sounds Assemble on the grassed area outside the front entrance of the LennardJones Laboratories re-entry to the building will not be allowed until Senior Fire Brigade Officer gives permission to do so YOU MUST NOT DO THESE Smoke or drink in the laboratory Drink the water from taps in the Laboratory – There are Drinking Water taps in the toilets Bring visitors into the laboratories AT ANY TIME Put bags or coats on the tables Remove the covers of any equipment or plugs, or use equipment with broken or damaged mains leads Work in the laboratory without supervision by staff YOU MUST DO THESE Store your bag/coats under the bench Report any suspected faults in equipment to the laboratory staff Error Bars and the Scientific Process (Lab Manual Section 2.2) Aim of Science? Advancement of knowledge, understanding nature, etc…. How is this achieved? Building theories/models and testing them by experimental measurements. Explaining the results of experiments by theoretical models. Comparing with theory/other experiments is clearly vital to the scientific process – but how to do this objectively? Error Bars and the Scientific Process An error bar DX signifies our “confidence” in a value that we measure X± DX “true” X lies within X- DX to X + DX ~66% “true” X lies within X- 2DX to X + 2DX ~95% “true” X lies within X- 3DX to X + 3DX ~99% Error Bars and the Scientific Process Error Bars and the Scientific Process Comparing two values Experiment and theory (X± DX) and (Y) Experiment and Experiment (X± DX) and (Y± DY) Does the value of X lie within the range of (Y± DY) or Does the value of Y lie within the range of (X± DX) If YES then X agrees with Y within the limits of error or vice versa Error Bars and the Scientific Process Examples 1. The SHM experiment – asked to compare the value of spring constant k measured from Hooke’s law with value measured from oscillations (SHM) 2. b-particle experiment – asked to compare measured value of E (determined from R via Feathers equation) with theoretical value of 2.26MeV Systematic and Random Errors Broad classification of errors into one of two classes systematic and random Systematic errors are the same every time you make a measurement. Random errors “fluctuate” every time you make a measurement Systematic Errors Zero offset errors in equipment Limits of measuring scales (e.g. Graduation on ruler/clock in SHM experiment) How to deal with systematic errors? Careful calibration, re-calibration of equipment. Use another piece of equipment to double check. Reduce scale graduation errors by measuring a larger value. e.g. In SHM experiment measure 10 oscillations instead of one, scale limit on clock is 1 sec whether measure 1 oscillation or 10. Use the graduation as error bar (sets limit to knowledge) or estimate of offset value (whichever is bigger). Random Errors The measured values fluctuate about a true value due to some random process, e.g. In b-particle experiment the count rate varies due to nuclear fluctuations. In electrical circuit “noise” can lead to fluctuations in measured voltages. In SHM experiment starting/stopping clock could be different each time you measure 10 oscillations. The effect of random errors can be treated statistically. Random Errors Measure N values x1, x2, x3,.....,xN • Mean value 1 x N N x i 1 i • Standard deviation for the fluctuations 1 2 ( xi x ) N 1 i 1 N • The error in the mean Dx N Combining and Propagating Error Bars We have measured a quantity in an experiment, and worked out its error bar ( call this raw data) Now we wish to process the value, i.e. use it in a formula. Since the raw value has an error bar, so must the “outcome” from the formula. Example, in SHM experiment you measure T, the periodic time and can get an error bar but what you need are values of T2, what is the error in T2 Even worse!!, in the b-particle experiment you measure the count rate for thickness of Al, get an error bar in each value and then you need the logarithm of the count rate. What is the error in the logarithm??? The propagation of Errors Through Functions ( X DX ) 2 ( X ) 2 2 X DX ( X DX ) n ( X ) n n X n 1 DX sin( X DX ) sin( X ) DX cos( X ) cos( X DX ) cos( X ) DX sin( X ) DX log e ( X DX ) log e ( X ) X log e (10) log10 ( X DX ) log10 ( X ) DX X exp( X DX ) exp( X ) DX exp( X ) 10 ( X DX ) 10 X DX log e (10)10 X Combing Errors Z X Y Z X Y DX DY DZ DX DY 2 2 2 2 2 2 2 DZ DX DY X Y Z Z X Y DZ DX DY X Y Z Z X Y XY Z UV DZ 2 DZ = Z 2 2 2 DX DY DU DV + + + X Y U V 2 Example A r 2 DA 2rDr DA 2Dr A r DA 2rDr 2 A r Fractional error in A = 2 x Fractional error in r or % error in A = 2 x % error in r In general A r n D A n Dr A r Fractional error in A = n x Fractional error in r or % error in A = n x % error in r Example A r 2 r 10.0 0.1m What is the value for A? A= x 10 x 10 = 314.16m2 Fractional error in r =0.1/10 Fractional error in A =2x0.1/10 =0.02 i.e. DA/A = 0.02 DA A x 0.02 = 314.16 x 0.02 =6.28m2 A 314 6m 2 Error Bars and Slopes/Intercepts From Graphs Plot a graph of raw data points, each one has an error bar. Want to fit a straight line, get slope, use slope in formula. Since the data points have error bar so the slope of the straight line will also have an error bar. “Theory” is called least squares data analysis. DON’T PANIC, leave the theory later. Computer program on PC’s in lab to fit straight lines and work out error bars. (LineFit / Excel / KyPlot / DataStudio) Templates for Excel or Kyplot is available on the web in the Physics/Astrophysics Laboratory web page.