Equations of State
advertisement

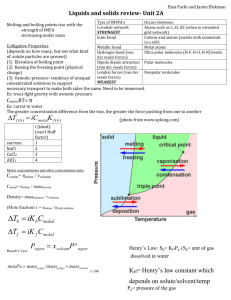
Equations of State Compiled by: Gan Chin Heng / Shermon Ong 07S06G / 07S06H How are states represented? Diagrammatically (Phase diagrams) Pressure Solid Liquid Critical point Triple point Gas Temp How are states represented? Mathematically Using equations of state Relate state variables to describe property of matter Examples of state variables Pressure Volume Temperature Equations of state Mainly used to describe fluids Liquids Gases Particular emphasis today on gases ABCs of gas equations Law B Boyle’s Law C Charles’ Law A Avogadro’s Avogadro’s Law At constant temperature and pressure Volume of gas proportionate to amount of gas i.e. V n Independent of gas’ identity Approximate molar volumes of gas dm3 at 298K 22.4 dm3 at 273K 24.0 Boyle’s Law At constant temperature and amounts Gas’ volume inversely proportionate to pressure, i.e. V 1/p The product of V & p, which is constant, increases with temperature Charles’ Law At constant pressure and amounts Volume proportionate to temperature, i.e. V T T is in Kelvins Note the extrapolated lines (to be explained later) Combining all 3 laws… V (1/p)(T)(n) V nT/p Rearranging, pV = (constant)nT Thus we get the ideal gas equation: pV = nRT Assumptions Ideal gas particles occupy negligible volume Ideal gas particles have negligible intermolecular interactions But sadly assumptions fail…Nothing is ideal in this world… It’s downright squeezy here Real gas particles DO occupy finite volume Real gas particles have considerable intermolecular interactions Failures of ideal gas equation Failure of Charles’ Law At very low temperatures Volume do not decrease to zero Gas liquefies instead Remember the extrapolated lines? Failures of ideal gas equation From pV = nRT, let Vm be molar volume pVm = RT pVm / RT = 1 pVm / RT is also known as Z, the compressibility factor Z should be 1 at all conditions for an ideal gas Failures of ideal gas equation Looking at Z plot of real gases… Obvious deviation from the line Z=1 Failure of ideal gas equation to account for these deviations So how? A Dutch physicist named Johannes Diderik van der Waals devised a way... Johannes Diderik van der Waals November 23, 1837 – March 8, 1923 Dutch 1910 Nobel Prize in Physics So in 1873… I can approximate the behaviour of fluids with an equation Scientific community ORLY? YARLY! Van der Waals Equation Modified from ideal gas equation Accounts for: Non-zero volumes of gas particles (repulsive effect) Attractive forces between gas particles (attractive effect) Van der Waals Equation Attractive effect Pressure = Force per unit area of container exerted by gas molecules Dependent on: Frequency of collision Force of each collision Both factors affected by attractive forces Each factor dependent on concentration (n/V) Van der Waals Equation Hence pressure changed proportional to (n/V)2 Letting a be the constant relating p and (n/V)2… Pressure term, p, in ideal gas equation becomes [p+a(n/V)2] Van der Waals Equation Repulsive effect Gas molecules behave like small, impenetrable spheres Actual volume available for gas smaller than volume of container, V Reduction in volume proportional to amount of gas, n Van der Waals Equation Let another constant, b, relate amount of gas, n, to reduction in volume Volume term in ideal gas equation, V, becomes (V-nb) Van der Waals Equation Combining both derivations… We get the Van der Waals Equation 2 n p + a [V-nb] = nRT V OR a p + 2 [Vm -b] = RT Vm Van der Waals Equation -> So what’s the big deal? Real world significances Constants a and b depend on the gas identity Relative values of a and b can give a rough comparison of properties of both gases Van der Waals Equation -> So what’s the big deal? Value of constant a Gives a rough indication of magnitude of intermolecular attraction Usually, the stronger the attractive forces, the higher is the value of a Some values (L2 bar mol-2): Water: 5.536 HCl: 3.716 Neon: 0.2135 Van der Waals Equation -> So what’s the big deal? Value of constant b Gives a rough indication of size of gas molecules Usually, the bigger the gas molecules, the higher is the value of b Some values (L mol-1): Benzene: 0.1154 Ethane: 0.0638 Helium: 0.0237 Critical temperature and associated constants Critical temperature? Given a p-V plot of a real gas… At higher temperatures T3 and T4, isotherm resembles that of an ideal gas Critical temperature? At T1 and V1, when gas volume decreased, pressure increases From V2 to V3, no change in pressure even though volume decreases Condensation taking place and pressure = vapor pressure at T1 Pressure rises steeply after V3 because liquid compression is difficult Critical temperature? At higher temperature T2, plateau region becomes shorter At a temperature Tc, this ‘plateau’ becomes a point Tc is the critical temperature Volume at that point, Vc = critical volume Pressure at that point, Pc = critical pressure Critical temperature At T > Tc, gas can’t be compressed into liquid At Tc, isotherm in a p-V graph will have a point of inflection 1st and 2nd derivative of isotherm = 0 We shall look at a gas obeying the Van der Waals equation VDW equation and critical constants Using VDW equation, we can derive the following a p + 2 [Vm -b] = RT Vm RT a p= - 2 Vm -b Vm VDW equation and critical constants At Tc, Vc and Pc, it’s a point of inflexion on pVm graph dp 0 dVm T d p 0 2 dVm T 2 VDW equation and critical constants dp RT 2a 3 2 (Vm b) Vm dVm T d2 p 2 RT 6a 4 2 3 dVm T (Vm b) Vm Rearranging... a 8a Vm,c = 3b; p c = ; Tc = 2 27b 27Rb p c Vm,c 3 Zc = = RTc 8 VDW equation and critical constants Qualitative trends As seen from formula, bigger molecules decrease critical temperature Stronger IMF increase critical temperature Usually outweighs size factor as bigger molecules have greater id-id interaction Real values: Water: 647K Oxygen: 154.6K Neon: 44.4K Helium: 5.19K Compressibility Factor Compressibility Factor Recall Z plot? Z = pVm / RT; also called the compressibility factor Z should be 1 at all conditions for an ideal gas Compressibility Factor For real gases, Z not equals to 1 Z = Vm / Vm,id Implications: At high p, Vm > Vm,id, Z>1 Repulsive forces dominant Compressibility Factor At intermediate p, Z < 1 Attractive forces dominant More significant for gases with significant IMF Boyle Temperature Z also varies with temperature At a particular temperature Z = 1 over a wide range of pressures That means gas behaves ideally Obeys Boyle’s Law (recall V 1/p) This temperature is called Boyle Temperature Boyle Temperature Mathematical implication Initial gradient of Z-p plot = 0 at T dZ/dp = 0 For a gas obeying VDW equation TB = a / Rb Low Boyle Temperature favoured by weaker IMF and bigger gas molecules Virial Equations Virial Equations Recall compressibility factor Z? Z = pVm/RT Z = 1 for ideal gases What about real gases? Obviously Z≠1 So how do virial equations address this problem? Virial Equations Form = 1 + B/Vm + C/Vm2 + D/Vm3 + … pVm/RT = 1 + B’p + C’p2 + D’p3 + … pVm/RT B,B’,C,C’,D & D’ are virial coefficients Temperature dependent Can be derived theoretically or experimentally Virial Equations Most flexible form of state equation Terms can be added when necessary Accuracy can be increase by adding infinite terms For same gas at same temperature Coefficients B and B’ are proportionate but not equal to each other Summary Summary States can be represented using diagrams or equations Ideal Gas Equation combines Avagadro's, Boyle's and Charles' Laws Assumptions of Ideal Gas Equation fail for real gases, causing deviations Van der Waals Gas Equation accounts for attractive and repulsive effects ignored by Ideal Gas Equation Summary Constants a and b represent the properties of a real gas A gas with higher a value usually has stronger IMF A gas with higher b value is usually bigger A gas cannot be condensed into liquid at temperatures higher than its critical temperature Summary Critical temperature is represented as a point of inflexion on a p-V graph Compressibility factor measures the deviation of a real gas' behaviour from that of an ideal gas Boyle Temperature is the temperature where Z=1 over a wide range of pressures Boyle Temperature can be found from Z-p graph where dZ/dp=0 Summary Virial equations are highly flexible equations of state where extra terms can be added Virial equations' coefficients are temperature dependent and can be derived experimentally or theoretically