Lab 1 Osmosis & Diffusion This diffusion lab write
advertisement

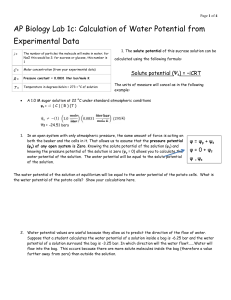
La This diffusion lab write-up sample is based on an older laboratory model. Introduction: Cells have kinetic energy. This causes the molecules of the cell to move around and bump into each other. Diffusion is one result of this molecular movement. Diffusion is the random movement of molecules from an area of higher concentration to areas of lower concentration. Osmosis is a special kind of diffusion where water moves through a selectively permeable membrane (a membrane that only allows certain molecules to diffuse though). Diffusion or osmosis occurs until dynamic equilibrium has been reached. This is the point where the concentrations in both areas are equal and no net movement will occur from one area to another. If two solutions have the same solute concentration, the solutions are said to be isotonic. If the solutions differ in concentration, the area with the higher solute concentration is hypertonic and the area with the lower solute concentration is hypotonic. Since a hypotonic solution contains a higher level of solute, it has a high solute potential and low water potential. This is because water potential and solute potential are inversely proportional. A hypotonic solution would have a high water potential and a low solute potential. An isotonic solution would have equal solute and water potentials. Water potential (y) is composed of two main things, a physical pressure component, pressure potential (yp), and the effects of solutes, solute potential (ys). A formula to show this relationship is y = yp + ys. Water will always move from areas of high water potential to areas of low water potential. The force of water in a cell against its plasma membrane causes the cell to have turgor pressure, which helps maintain the shape of the cell. When water moves out of a cell, the cell will loose turgor pressure along with water potential. Turgor pressure of a plant cell is usually attained while in a hypotonic solution. The loss of water and turgor pressure while a cell is in a hypertonic solution is called plasmolysis. Hypothesis: During these experiments, it will be proven that diffusion and osmosis occur between solutions of different concentrations until dynamic equilibrium is reached, affecting the cell by causing plasmolysis or increased turgor pressure during the process. Materials: Lab 1A - To begin Lab 1A, first collect the desired equipment. The materials needed are dialysis tubing, Iodine Potassium Iodide (IKI) solution, 15% glucose/ 1% starch solution, glucose Testape or Lugol’s solution, distilled water, and a 250-mL beaker. Lab 1B - For Lab 1B you will need to collect six presoaked dialysis tubing strips, distilled water; 0.2M, 0.4M, 0.6M, 0.8M, and a 1.0M sucrose solution; six 250-mL beakers or cups, and a scale. Lab 1C - Lab 1C these items are needed: a potato, knife, potato core borers, six different solutions, and a scale. Lab 1D During Lab 1D, only paper, pencil, and a calculator will be needed to make the calculations. Lab 1E n Lab 1E these items are needed: a microscope slide, cover slip, onion cells, light microscope, and a 15% NaCl solution. Procedures: Lab 1A - After gathering the materials, pour glucose/starch solution into dialysis tubing and close the bag. Test the solution for presence of glucose. Test the beaker of distilled water and IKI for presence of glucose. Put the dialysis bag into the beaker and let stand for 30 minutes. When time is up test both the bag and the beaker for presence of glucose. Record all data in table. Lab 1B - Obtain the six strips of dialysis tubing and fill each with a solution of a different molarity. Mass each bag. Put each bag into a beaker of distilled water and let stand for half and hour. After 30 minutes is up, remove each bag and determine its mass. Record all data in its appropriate table. Lab 1C - sing the potato core borer, obtain 24 cylindrical slices of potato, four for each cup. Determine the mass of the four cylinders. Immerse four cylinders into each of the six beakers or cups. Let stand overnight. After time is up, remove the cores from the sucrose solutions and mass them. Record all data in its appropriate table. Lab 1D - Using the paper, pencil, and calculator collected, determine solute potentials of the solutions and answer the questions asked to better understand this particular part of the lab. Lab 1E - Using the materials gathers, prepare a wet mount slide of the epidermis of an onion. Draw what you see of the onion cell under the microscope. Add several drops of the NaCl solution to the slide. Now draw the appearance of the cell. Data: Lab 1A - Table 1.1 Contents Bag Beaker 15% Glucose/ 1% Starch Solution H2O+IKI Initial Color clear Final Color Initial Presence of Glucose Dark blue + Orange to brown Final Presence of Glucose Orange to brown _ + + Lab 1A Questions 1) Glucose is leaving the bag and Iodine-Potassium-Iodide is entering the bag. The change in color of the contents of the bag and the presence of glucose in the bag prove this. 2) In the results, the IKI moved from the beaker to the bag, this caused the change in the color of the bag. The IKI moved into the bag to make the concentrations outside the bag equal to inside the bag. The glucose solution moved out of the bag making glucose present in the beaker. The glucose moved to make the solute concentration inside and out of the bag equal. 3) If the initial and final percent concentration of glucose and IKI for in the bag and the beaker were given, they would show the differences and prove the movement of these substances to reach dynamic equilibrium. 4) Based on my observations, the smallest substance was the IKI molecule, then the glucose molecules, water molecules, membrane pore, and then the starch molecules being the largest. 5) If the experiment started with glucose and IKI inside the bag and starch in the beaker, the glucose and IKI would move out of the bag to make the concentrations equal, but the starch could not move into the bag because its molecules are too big to pass through the semipermeable membrane. Lab 1B - Table 1.2 Dialysis Bag Results Contents in dialysis bag Initial mass Final mass Mass difference Percent change in mass a) distilled water 26.5g 26.6g 0.1g 0.4% b)0.2M 28.1g 29.3g 1.2g 4.3% c)0.4M 27.3g 30.1g 2.8g 10.3% d)0.6M 28.3g 32.3g 4.0g 14.1% e)0.8M 25.9g 30.7g 4.8g 18.5% f)1.0M Table 1.3 26.7g 32.9g 6.2g 23.2% Dialysis Bag Results: Class Data Group 1 Group 2 Group 3 Group 4 Total Class Average Distilled Water 0.4% 1.16% 0.79% 1.54% 3.89% 1.0% 0.2M 4.3% 5.99% 6.44% 5.94% 22.67% 5.67% 0.4M 10.3% 10.49% 10.33% 8.45% 39.57% 9.89% 0.6M 14.1% 14.86% 16.04% 15.1% 60.1% 15.03% 0.8M 18.5% 19.80% 17.97% 20.0% 76.27% 19.07% 1.0M 23.2% 18.77% 23.55% 21.9% 87.42% 21.86% Lab 1B Questions: 1) The molarity of the sucrose in the bag determines the amount of water that either moves into or out of the bag, which changes the mass. For example, when the bag contained a 0.2M solution, water entered the bag to make the concentrations inside and outside of the bag more equal. As this happened, the mass rose 1.2g 2) If each of the bags were placed into a 0.4M solution instead of distilled water, the masses of the bags would have changed in different ways. The mass of the bags filled with distilled water and 0.2M sucrose would have gone down because water would have left the bag. The mass of the 0.4M bag would have stayed the same because the concentrations are now equal. The masses of the 0.6, 0.8, and 1.0M bags would have increased because water would have moved into the bag to equalize the concentrations. 3) In the data collected, the percent change in mass was calculated to show how greatly the mass increased or decreased. The difference in mass is not enough to go by because the initial masses of the dialysis bags were not all the same. 4) If a dialysis bag’s initial mass was 20g and it’s final mass was 18g, the percent change in mass is 20%. 5) The sucrose solution in the beaker would have been hypotonic to the distilled water in the bag. Lab 1C Table 1.4 Contents of Beaker Initial Mass Final Mass Difference in Mass % Change in Mass Initial Temp. Final Temp. Distilled Water 1.5g 2.0g 0.5g 33% 20°C 20°C 0.2M 1.5g 1.6g 0.1g 7% 21°C 20°C 0.4M 1.5g 1.6g 0.1g 7% 20°C 20°C 0.6M 1.5g 1.5g 0.0g 0% 21°C 20°C 0.8M 1.5g 1.2g -0.3g -20% 21°C 20°C 1.0M 1.5g 1.4g -0.1g -7% 20°C 20°C Lab 1C Table 1.5 Class Results Percent Change in Mass of Potato Cores Group 1 Group 2 Group 3 Group 4 Distilled Water 33% 35.29% 25% 31.25% 0.2M 7% 29.41% 25% 13.33% 0.4M 7% 11.11% -12.5% -12.5% 0.6M 0% -15.79% -18.75% -20% 0.8M -20% -15.79% -18.75% -25% 1.0M -7% 0% -18.75% -20% Lab 1 D Questions: 1) The water potential of the potato core after dehydrating will decrease because the water within the potato would evaporate and therefore lower the water potential. 2) The solute concentration of the plant cell is hypertonic because the solute concentration is higher than the water concentration. Because of this, water will diffuse into the cell to reach dynamic equilibrium. 3) The pressure potential of the system is equal to 0. 4) The water potential is greater in the dialysis bag. 5) Water will diffuse out of the bag since the water potential is higher in the bag and water moves from areas of higher water potential to areas of lower water potential. 6) Zucchini cores placed in sucrose solutions at 27°C resulted in the following percent changes after 24 hours: Percent Change in Mass 20% 10% -3% -17% -25% -30% 8)ys=-iCRT ys=-(1)(0.35)(0.0831)(295) ys=-8.580075 y=0+ys y=0+(-8.580075) y=-8.580075 9) Adding solute to a solution increases solute potential because the solute concentration increases. 10) The distilled water would have a higher concentration of water molecules and would also have a higher water potential. The red blood cells would increase in size because water is moving from the area of higher water potential (the distilled water) to the area of lower water potential (the red blood cells) until dynamic equilibrium is reached. Lab 1E Questions 1) After preparing a wet mount slide, I have observed the onion cells under magnification and they appear to be small, empty boxes pushed closely together. 2) By adding two or three drops of NaCl the cells should have shrunk, but no change took place. 3) The cells maintained the same shape. 4) Plasmolysis is the lose of water and turgor pressure in a cell. 5) The onion cells should have plasmolyzed because the area surrounding them had a lower water potential and water should have moved out of the cells. 6) Grasses that live on the sides of roads that have been salted in the winter end to dies because the water is drained from the cells as it moves out of the grass cells into the hypertonic NaCl area around it. Lab 1D Plasmolysis of Cells - Drawings of onion cells 100X Onion Cells in Distilled Water *Picture Of onion cells in saline not available. Error Analysis: Lab 1A - The data collected in this lab experiment did not seem to contain any inconsistencies, so therefore no human error is detected. Lab 1B - In this lab experiment, the data seems to be compliant with the data collected by the other lab groups, so no human error was thought to have happened. Lab 1C - There was some discrepancy in this experiment in the 1.0M solution’s percent change in mass of potato cores. The data decreases consistently until the 1.0M solution, so human error is thought to be a factor in this. Some mistakes that could have taken place are miscalculations in initial and final masses or problems with the molarity of the solution itself. Lab 1D - In this part of the lab, only calculations were made, so no human error probably occurred during this time. Lab 1E - In part 1E, after adding the NaCl solution to the onion cells, the cells should have reduced in size, but no reaction took place. This may have occurred in part because the onion itself was already dried out and dehydrated, or while the onion was being looked at through the microscope, the heat from it may have caused the cells to loose water. Conclusion: During the experiment conducted in Lab 1A, the results and data collected make it possible to conclude that glucose and Iodine Potassium Iodide can pass through a selectively permeable membrane and will if the concentrations on either side are not equal. In Lab 1B, it can be concluded that sucrose cannot pass over a selectively permeable membrane, but instead water molecules will move across the membrane to the area of lower water potential to reach dynamic equilibrium. Lab 1C provided information that helps to conclude that potatoes do contain sucrose molecules. This can be stated because the cores took in water while they were emerged in the distilled water. This means they had a lower water potential and higher solute potential than the distilled water. The solute potential is equal to about a 0.6M solution of sucrose according to the data collected. During Lab 1D calculations were made and questions were answered to help give a better understanding of water and solute potential. If the onion cell experiment in part 1E of the lab would have produced correct results, conclusions could have been made. It is thought that the onion cells would have plasmolyzed due to the addition of NaCl to the cells. This shows how the onion cells had high water potential and moved to the area outside the cell with lower water potential. Then, after adding water back to the cells, water would have moved back into the cells increasing turgor pressure. The water potential played an enormous role in each part of this lab. Since water moves areas of high water potential to areas of low water potential, reactions took place in each part resulting in different conclusions being derived from them. Water potential was a key element in each part of the experiment. In plant and animal cells, loss or gain of water can have different effects. In a plant cell, it is ideal to have an isotonic solution. If the solution is hypertonic, the cell will shrivel from lack of water intake. Inversely, if the solution is hypotonic the cell could take in too much water and the cell will lyse and break open. For a plant cell, the ideal solution is a hypotonic solution because the cell takes in water increasing turgor pressure which keeps the cells tightly packed and keep their shape. If the solution is hypertonic, the cell will plasmolyze and died from lack of water. In an isotonic solution, the plant cell does not have enough turgor pressure to keep is shape.