Mitosis Lab: Allium Root Tip Squash Protocol
advertisement

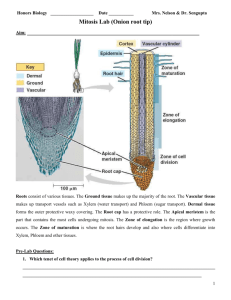
DETERMINING THE TIME NEEDED FOR MITOSIS IN A LIVING ROOT: ALLIUM ROOT TIP SQUASH PREPARATION INTRODUCTION: Think about your theoretical knowledge and your past practical work. What question are you investigating? What makes this investigation relevant? interesting? HYPOTHESIS: Based on your previous lab activity, what answer(s) to your questions do you predict? Remember to use the “If...then...” format. MATERIALS AND METHODS: PART A: GROWING A ROOT FOR MAKING THE SQUASH SLIDE There is more than one type of plant root that can be used. No matter what type of plant you use, be aware that not every squash slide produces a usable slide. It may take a few tries. That being the case, you should ensure that you have at least 10 roots. Try to start this at least 4 days before the lab day. Note the date that you began the rooting procedure and the day the roots first begin to grow. Onion bulb or garlic: *Of these, garlic is often recommended (see Reference 1 below.). Figure 1. A garlic clove suspended in water. http://www.microscopyuk.org.uk/mag/indexmag.html?http://www.microscopyuk.org.uk/mag/artdec06/yl-squash.html 1. Remove the dried roots from the onion or garlic bulb. 2. For garlic, one or more cloves is all that is necessary. 3. If possible, find a tube or container that can hold the bulb/clove so that the bottom just touches the surface of the water in the container. Strong toothpicks or grilling skewers can be inserted into the bulb/clove to hold it up if the container’s diameter is too large. Green/spring onion: 1. Remove the old roots with a sharp knife. 2. Place in water. The green onion does not need to be suspended in the way that the large onion bulb or garlic clove does. 1 Sprouted seeds: 1. Moisten a paper towel and place it on a tray or in a plastic bag such as a “Ziploc” bag. The paper towel should feel damp but not drip. 2. Place the seeds on the paper towel. 3. Cover with another moist paper towel. If on a tray, cover the entire setup with plastic wrap. Do not seal the plastic wrap to the tray too tightly so that air can still circulate. If in a plastic bag, seal the bag but leave space for air to circulate. 4. Maintain the moistness of the paper towels but do not make the setup too wet because mould may grow. Check approximately every 12 hours. PART B: PREPARING A SQUASH SLIDE OF A PLANT ROOT *Wear goggles until the slide is ready to view because we are using a variety of chemicals. 250 or 400 mL beaker Hotplate 2 watch glasses/petri dishes Paper towel Cover slip 50-100 mL beaker test tube razor blade/scalpel microscope thermometer test tube holder forceps/tweezers slide HCl acetocarmine stain/toluidine blue stain 1. Prepare a 60C water bath: a. Get a 250 mL or 400 mL beaker about half full of water and put it on a hot plate. b. Put a thermometer in the beaker. c. Turn on the hot plate to high. d. When the thermometer reading is about 50C, turn down the hot plate to a the lowest setting. The water will continue to heat. e. Maintain the temperature at about 60 C by adding a little cold water if the water gets too hot. f. Up to 4 test tubes can use one hot water bath. Figure 2. Cutting the end of the roots off an onion. (same source as Fig. 1) 2 2. Obtain onion/garlic/green onion/sprouted seeds with roots. Cut off 2-3 roots such that they are at least 1.5 cm long. This is so that they will be easier to manipulate for the fixing and staining part of the process. 3. To “soften” the roots so that they can be spread: a. Put the root tips into a clean test tube and add just enough hydrochloric acid (HCl) to cover. We are using 1M HCl which is quite a concentrated solution of a strong acid. b. Put the test tube into a 60C water bath and leave for 10 minutes. 4. Rinse the roots in water: a. b. c. d. e. f. Pour the test tube out onto a watch glass or petri dish. Using the forceps, transfer the roots to a second clean watch glass or petri dish. Using a clean beaker, gently add a small amount of water to cover the root tips. Meanwhile, rinse off the first watch glass/petri dish. Transfer the root tips to the newly clean watch glass/petri dish. Repeat c-e two more times for a total of 3 rinses. 5. Staining the root tips: Be careful not to touch the stain or get it on your clothes. a. Gently blot the root tips with a paper towel. Figure 3. How to blot. http://www.carolina.com/category/teacher+resources/classroom+activities/squ ash+an+onion.do Figure 4. Root tips after staining. Same source as Figure 1. b. Add either acetocarmine stain or toluidine blue stain to the root tip on the watch glass/petri dish. Leave for 2 minutes. Remove the root tip to a clean watch glass/petri dish and blot with a paper towel. c. d. 3 6. Finally...the squashing! a. Cut 1-2 mm off the root tip. It should be stained a darker colour than the rest of the root. b. Transfer the root tip to a slide. c. Cover with a cover slip. d. Place a small piece of paper towel on the cover slip. e. Using your thumb or the eraser on the end of a pencil, press down on the cover slip where the root tip is. Press hard! DO NOT TWIST AT ALL! The root tip should spread to about 5-10mm. If needed, press again. Figure 5. The finished slide. Same source as Figure 1. 7. View the slide. See Results below to make sure that you have a complete set of observations. RESULTS: 1. Draw one example of a cell in each of the following stages of the life cycle: Interphase, Prophase, Metaphase, Anaphase and Telophase. 2. Perform a cell count just as you did in the earlier lab and perform the relevant calculations to determine the time needed for mitosis. 3. Add your data to the class data on the class computer. DISCUSSION: 1. Compare the images you saw with the prepared slides and the images you see with your squash slide. 2. How did your individual results for time needed for mitosis compare to the results you got using the prepared slides of Allium and whitefish blastula? 3. How did your individual results compare to the class results? 4. In the mitosis lab with prepared slides and this lab, you were asked to look at your individual results and class results. Why? What are the advantages and disadvantages of using just individual or just class data? How does this relate to experimental design in general? 4 CONCLUSION: Evaluate the methods including any sources of error. Make a final statement about the time needed for mitosis based on your results from these two labs. Find a reputable source that gives the time needed for the entire cell cycle and the individual stages (look for Interphase rather than its separate stages) and compare your results to those given. Don’t forget to include the source in your writeup. A formal reference section is not needed. REFERENCES: 1. For tips on obtaining roots and a common procedure for fixing, staining and squashing the root sample: http://www.practicalbiology.org/areas/advanced/cells-to-systems/celldivision/investigating-mitosis-in-allium-root-tip-squash,121,EXP.html 2. A complete protocol for a mitosis lab including estimating the time for mitosis: http://www.marietta.edu/~biol/introlab/Onion%20root%20mitosis.pdf 3. A complete protocol for a mitosis lab: http://faculty.valenciacollege.edu/tklenk/labs/mitosislab.htm 4. Another complete protocol: http://www.carolina.com/category/teacher+resources/classroom+activities/sq uash+an+onion.do 5. Complete protocol: http://www.microscopyuk.org.uk/mag/indexmag.html?http://www.microscopyuk.org.uk/mag/artdec06/yl-squash.html 5