Molarity Problems Worksheet
advertisement
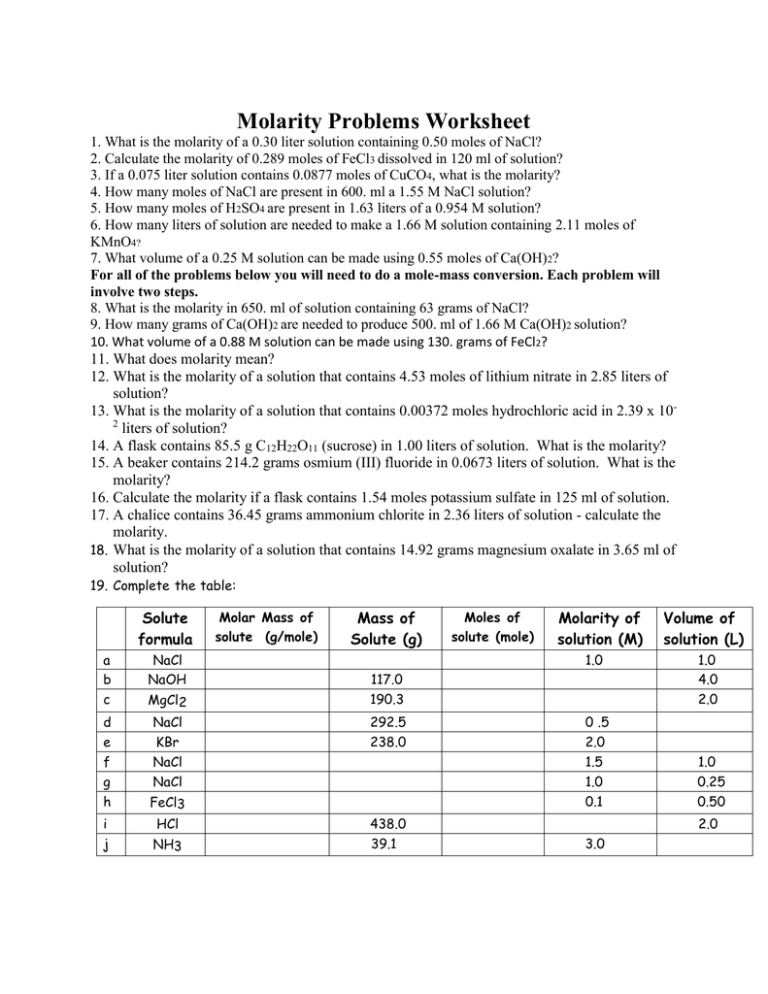
Molarity Problems Worksheet 1. What is the molarity of a 0.30 liter solution containing 0.50 moles of NaCl? 2. Calculate the molarity of 0.289 moles of FeCl3 dissolved in 120 ml of solution? 3. If a 0.075 liter solution contains 0.0877 moles of CuCO4, what is the molarity? 4. How many moles of NaCl are present in 600. ml a 1.55 M NaCl solution? 5. How many moles of H2SO4 are present in 1.63 liters of a 0.954 M solution? 6. How many liters of solution are needed to make a 1.66 M solution containing 2.11 moles of KMnO4? 7. What volume of a 0.25 M solution can be made using 0.55 moles of Ca(OH)2? For all of the problems below you will need to do a mole-mass conversion. Each problem will involve two steps. 8. What is the molarity in 650. ml of solution containing 63 grams of NaCl? 9. How many grams of Ca(OH)2 are needed to produce 500. ml of 1.66 M Ca(OH)2 solution? 10. What volume of a 0.88 M solution can be made using 130. grams of FeCl2? 11. What does molarity mean? 12. What is the molarity of a solution that contains 4.53 moles of lithium nitrate in 2.85 liters of solution? 13. What is the molarity of a solution that contains 0.00372 moles hydrochloric acid in 2.39 x 102 liters of solution? 14. A flask contains 85.5 g C12H22O11 (sucrose) in 1.00 liters of solution. What is the molarity? 15. A beaker contains 214.2 grams osmium (III) fluoride in 0.0673 liters of solution. What is the molarity? 16. Calculate the molarity if a flask contains 1.54 moles potassium sulfate in 125 ml of solution. 17. A chalice contains 36.45 grams ammonium chlorite in 2.36 liters of solution - calculate the molarity. 18. What is the molarity of a solution that contains 14.92 grams magnesium oxalate in 3.65 ml of solution? 19. Complete the table: Solute formula Molar Mass of solute (g/mole) Mass of Solute (g) Moles of solute (mole) Molarity of solution (M) a b c NaCl NaOH MgCl2 117.0 190.3 d NaCl 292.5 0 .5 e f g h KBr NaCl NaCl 238.0 2.0 1.5 1.0 0.1 i j FeCl3 HCl NH3 1.0 438.0 39.1 Volume of solution (L) 1.0 4.0 2.0 1.0 0.25 0.50 2.0 3.0 20) Calculate the number of moles and the number of grams of solute in each solution: Moles of solute (mol) a 1.0 liter of .5 M NaCl b 500.0 mL of 2.0 M KNO3 c 250.0 mL of .10 M CaCl2 d 2.0 liters of .30 M Na2SO4 Molar Mass of solute (g/mol) Mass of solute (g) 21) Compute the mass of solute needed to make 500.0 mL of solution at the indicated molarity. a: 0.5M sulfuric b: 0.1M Iron III chloride c: 0.01M HNO3 d: 3M NH3 e: 6.0M HCl f: 5.0M KOH g: 0.50M sodium hydroxide 22) How many moles of H2SO4 are in 1.00 liter of a 1.55M H2SO4 solution? How many grams? 23) How many grams of sodium sulfate are contained in 1.50L of .25M solution? 24) How many grams of ammonium sulfate are required to prepare 3.50L of a 1.55M solution? 25) How many moles of sodium chromate are contained in 1.75 L of a 2.00M solution? 26) A sample of glucose (C6 H12O6) is dissolved in water. How many moles of glucose are dissolved in 200.0 ml of solution if its concentration is .150M? 27) A mass of 98g of sulfuric acid are dissolved in water to prepare a .50M solution. What is the volume of the solution, in liters? 28) What is the molarity of a solution of HNO3 that contains 12.6g of solute in 500ml of solution?






