Chem 10-11-116
advertisement

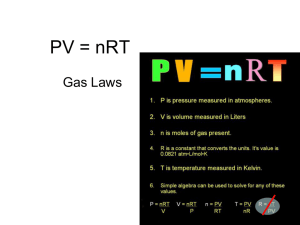
A student is working with a sample of nitrogen gas. The gas is contained in a cylinder with a volume of 5.0 liters, and is under a pressure of 3.5 atmospheres. The student carefully transfers the gas sample into a 7.0 liter container. What will the pressure on the gas be in its new container? What law governs this? 2.5 atm Boyles’ Law Announcements ?? Six-Week’s Assignment List Date Issued Date Due WS - The Kinetic Molecular Theory and Nature of Gases 2/24 3/3 Quiz – Pressure Unit Conversions 3/3 3/3 WS – The Gas Laws 3/3 3/10 Continue Worksheet The Gas Laws The Gas Laws • Boyle’s Law pressure – volume relationships • Charles’s Law volume - temperature relationships • Gay-Lussac’s Law pressure – temperature relationships • The Combined Gas Law volume – temperature – pressure changes • Dalton’s Law of Partial Pressures partial pressures and total pressures Charles’ Law • Volume - Temperature relationships • The relationship between volume and temperature was discovered by the French scientist Jacques Charles in 1787. • Charles’s experiments showed that all gases expand to the same extent when heated through the same temperature interval. Charles’ Law • Charles found that the volume changes by 1/273 of the original volume for each Celsius degree, at constant pressure and an initial temperature of 0°C. – Ex) Raising the temperature to 1°C causes the gas volume to increase by 1/273 of the volume it had at 0°C. – A 10°C temperature increase causes the volume to expand by 10/273 of the original volume at 0°C. – If the temperature is increased by 273°C, the volume increases by 273/273 of the original, that is, the volume doubles. Charles’ Law • The same regularity of volume change occurs if a gas is cooled at constant pressure. – At 0°C, a 1°C decrease in temperature decreases the original volume by 1/273. – At this rate of volume decrease, a gas cooled from 0°C to -273°C would be decreased by 273/273. – In other words, it would have zero volume, which is not actually possible. Charles’ Law • In fact, real gases cannot be cooled to 273°C - before they reach that temperature, intermolecular forces exceed the kinetic energy of the molecules, and the gases condense to form liquids or solids. • The Kelvin temperature scale is a scale that starts at a temperature corresponding to 273.15°C. • That temperature is the lowest one possible. Charles’ Law • The temperature -273.15°C is referred to as absolute zero and is given a value of zero in the Kelvin scale. • This fact gives the following relationship between the two temperature scales: K = °C + 273 For calculations in this book, 273.15 is rounded off to 273. • The average kinetic energy of gas molecules is more closely related to the Kelvin temperature. Charles’ Law • Gas volume and Kelvin temperature are directly proportional to each other. – Ex) Quadrupling the Kelvin temperature causes the volume of a gas to quadruple – Reducing the Kelvin temperature by half causes the volume of a gas to decrease by half. • The relationship between Kelvin temperature and gas volume is known as Charles’s law. Charles’ Law • Charles’s law states that the volume of a fixed mass of gas at constant pressure varies directly with the Kelvin temperature. • Charles’s law may be expressed as follows. V = kT or V/T = k – The value of T is the Kelvin temperature – k is a constant - the value of k depends only on the quantity of gas and the pressure. – The ratio V/T for any set of volumetemperature values always equals the same k. Charles’ Law • The form of Charles’s law that can be applied directly to most volume-temperature problems involving gases is as follows. V1/T1 = V2/T2 – V1 and T1 represent initial conditions. – V2 and T2 represent a new set of conditions. – When three of the four values V1, T1, V2 , and T2 are known, this equation can be used to calculate the fourth value. Charles’ Law Sample Problem • A sample of neon gas occupies a volume of 752 mL at 25°C. What volume will the gas occupy at 50°C if the pressure remains constant? • Unknown is V2 of Ne in mL V1/T1 = V2/T2 V2 = V1T2/T1 V2 = (752 mL Ne)(323K)/298K V2 = 815 mL Ne Gay-Lussac’s Law • Pressure - Temperature relationships • For a fixed quantity of gas at constant volume, the pressure is directly proportional to the Kelvin temperature, which depends directly on average kinetic energy. • For every kelvin of temperature change, the pressure of a confined gas changes by 1/273 of the pressure at 0°C. Gay-Lussac’s Law • Joseph Gay-Lussac is given credit for recognizing this in 1802. • Gay-Lussac’s law: The pressure of a fixed mass of gas at constant volume varies directly with the Kelvin temperature. P = kT, or P/T = k – The value of T is the temperature in kelvins – k is a constant that depends on the quantity of gas and the volume. Gay-Lussac’s Law • For a given mass of gas at constant volume, the ratio P/T is the same for any set of pressure-temperature values. • Unknown values can be found using this form of Gay-Lussac’s law: P1/T1 = P2/T2 • When values are known for three of the four quantities, the fourth value can be calculated. Gay-Lussac’s Law Sample Problem • The gas in an aerosol can is at a pressure of 3.00 atm at 25°C. Directions on the can warn the user not to keep the can in a place where the temperature exceeds 52°C. What would the gas pressure in the can be at 52°C? • Unknown is P2 of gas in atm. P1/T1 = P2/T2 P2 = P1T2/T1 P2 = (3.00 atm)(325K)/298K P2 = 3.27 atm