2 (aq)

Types of Chemical
Reactions and Solution
Stoichiometry
AP Chemistry
Mrs. Weston
Chapter 4
1
Properties of Solutes in Aqueous Solution
Water, the Common Solvent
Solute: substance dissolved in a liquid to form a solution
-Dissolve in the solvent (often water)
-Is present in lesser amount than the solvent
Solvent: the dissolving medium
When water is the solvent the solution is aqueous
- abbreviated (aq)
2
Properties of Solutes in Aqueous Solution
Water, the Common Solvent
Water is a polar molecule
•
Since Oxygen is slightly more electronegative than
Hydrogen, it obtains a partial
- charge; hydrogen receives a partial + charge
• The positive end of the water molecule is attracted to the negatively charged solute ions.
• This process is called hydration
3
Properties of Solutes in Aqueous Solution
Ionic Compounds in Water
Ions dissociate in water.
In solution, each ion is surrounded by water molecules.
Transport of ions through solution causes flow of current
Video
4
Properties of Solutes in Aqueous Solution
Molecular Compounds in Water
Molecular compounds in water (e.g., CH
3
OH): no ions are formed.
If there are no ions in solution, there is nothing to transport electric charge.
5
Properties of Solutes in Aqueous Solution
Strong and Weak Electrolytes
Strong electrolytes: completely dissociate in solution.
For example:
HCl(aq) H
+
(aq) + Cl
-
(aq)
Weak electrolytes: produce a small concentration of ions when they dissolve.
These ions exist in equilibrium with the un-ionized substance. For example:
HC
2
H
3
O
2
(aq) H
+
(aq) + C
2
H
3
O
2
-
(aq)
6
Properties of Solutes in Aqueous Solution
Strong and Weak Electrolytes
7
Properties of Solutes in Aqueous Solution
Acids
Strong acids -dissociate completely to produce H + in solution ex: hydrochloric and sulfuric acid
Weak acids - dissociate to a slight extent to give H + in solution ex: acetic and formic acid
8
Properties of Solutes in Aqueous Solution
Bases
Strong bases - react completely with water to give OH ions.
ex: sodium hydroxide
Weak bases - react only slightly with water to give OH ions.
ex: ammonia
9
The Composition of Solutions
Often when performing stoichiometric calculations we need to know how much of a reagent is found in solution.
• usually expressed as a concentration
M
molarity
moles of solute liters of solution
3
M
HCl
6 moles of HCl
2 liters of solution
10
The Composition of Solutions
Problem 1: If 1.56 g of gaseous HCl is dissolved in enough water to form 26.8 ml of solution, what is the molarity?
11
The Composition of Solutions
Problem 2: Typical blood serum is about 0.14 M NaCl.
What volume of blood contains 1.0 mg of NaCl?
12
The Composition of Solutions
Preparing Dilutions a) Mass the appropriate amount of solid substance
(the solute), then place the solute in a volumetric flask b) Add approx. 1/3 of the total volume of solvent required
13
The Composition of Solutions
Preparing Dilutions c) Dissolve the solute by swirling the flask
(with stopper in place) d) Add water until the level of the solution just reaches the etched mark on the flask, then invert the flask 7x
14
The Composition of Solutions
Preparing Dilutions
(a) A measuring pipet is graduated and can be used to measure various volumes of liquid accurately.
(b) a volumetric (transfer) pipet is designed to measure one volume accurately.
Video
– Types of Pipets
Video
– Creating a dilution
15
The Composition of Solutions
Preparing Dilutions
Problem 3: To analyze the alcohol content of a certain wine, a chemist needs 0.750 L of an aqueous 0.200 M potassium dichromate solution.
How much solid must be weighed out to make the solution? Describe how the solution would be prepared.
16
The Composition of Solutions
Preparing Dilutions
Problem 4: When preparing acid solutions for the lab, it is often necessary to dilute a concentrated stock solution.
If 1.5 L of 0.50 M H
2
SO
4 solution is needed, and the H
2
SO
4 stock solution is 16 M, describe how to prepare the dilute solution.
17
Types of Chemical Reactions
Major Categories of Chemical Reactions
• Precipitation reactions
AgNO
3
( aq ) + NaCl( aq )
AgCl( s ) + NaNO
3
( aq )
•
Acid-base reactions
NaOH( aq ) + HCl( aq )
NaCl( aq ) + H
2
O( l )
•
Oxidation-reduction reactions
Fe
2
O
3
( s ) + Al( s )
Fe( l ) + Al
2
O
3
( s )
18
Precipitation Reactions
Two solutions are mixed yielding an insoluble substance called a precipitate
Ex: K
2
CrO
4
(aq) + Ba(NO
3
)
2
(aq) --> products
In order to determine which product yields the precipitate we need to consider each of the ions available in the reaction
19
Precipitation Reactions:
Writing Ionic Equations
1) Write the
molecular equation
- shows all species listed in their molecular forms:
Ex: K
2
CrO
4
(aq) + Ba(NO
3
)
2
(aq) -->
BaCrO
4
(s) + 2 KNO
3
(aq)
2) Write the
Complete ionic equation
List all ions and solid products (in a complete ionic equation, all strong electrolytes are written as ions)
Ex: 2K + (aq) + CrO
4
2(aq) + Ba 2+ (aq) + 2 NO
3
(aq) -->
BaCrO
4
(s) + 2 K + (aq) + 2NO
3
(aq)
20
Precipitation Reactions:
Writing Ionic Equations
3) Write the
Net ionic equation
-list only the unique ions which are found in ion form on only one side of the equation. Also write any solid or gaseous reagents
Ex: CrO
4
2(aq) + Ba 2+ (aq)
--> BaCrO
4
(s)
Spectator ions: K + (aq) & NO
3
(aq)
21
Simplified Solubility Rules
Memorize these!!
Video
Lyrics to song
1. Most compounds containing Group 1 elements and the ammonium ion are soluble.
2. Most nitrates, chlorates, and acetates are soluble, except silver acetate.
3. Most halogen salts are soluble except those of silver, mercury (I), and lead.
4. Most sulfates are soluble, except those of silver, mercury (I or II), lead, calcium, strontium, and barium.
5. Calcium, strontium, and barium hydroxides are soluble, along with group 1 and ammonium. Most other hydroxides are basically insoluble.
6. Ionic compounds not covered by a previous rule are mostly insoluble.
22
Using Solubility Rules
23
Using Solubility Rules
Problem: Predict what, if anything, will happen when the following pairs of solutions are mixed: a. KNO
3
(aq) and BaCl
2
(aq) b. Na
2
SO
4
(aq) and Pb(NO
3
)
2
(aq) c. KOH (aq) and Fe(NO
3
)
3
(aq)
24
Using Solubility Rules
Problem: Predict what, if anything, will happen when solutions of potassium hydroxide and iron
(III) nitrate are mixed. If any reaction occurs, write the molecular equation & net ionic equation.
25
Stoichiometry of Precipitation Reactions
26
Stoichiometry of Precipitation Reactions
Use your knowledge of stoichiometry from Chapter 3 to solve the following problem:
When 2.00 L of 0.0250 M sodium sulfate and 1.25 L of
0.0500 M lead (II) nitrate are mixed, what substance precipitates? How many grams of that substance will be formed?
27
Introduction to Acid/Base Reactions
Acid/Base Reactions
Steps for solving problems:
1.
List initial species and predict reaction.
2.
Write balanced net ionic reaction.
3.
Calculate moles of reactants.
4.
Determine limiting reactant.
5.
Calculate moles of required reactant/product.
6.
Convert to grams or volume, as required.
28
Introduction to Acid/Base Reactions
Acid/Base Reactions
In a certain experiment, 28.0 ml of 0.250 M HNO
3 and 53.0 ml of 0.320 M KOH are mixed. Calculate the amount of water formed. What is the concentration of H + or OH ions in excess after the reaction goes to completion?
29
Introduction to Acid/Base Reactions
Strong Acids
•
HCl
•
HBr
•
HI
• HNO
3
• H
2
SO
4
• HClO
3
•
HClO
4
Strong Bases
•
LiOH
•
NaOH
•
KOH
• RbOH
• Ca(OH)
2
• Sr(OH)
2
•
Ba(OH)
2
30
Introduction to Oxidation-Reduction
Reactions
Oxidation and Reduction
•
When a metal undergoes corrosion it loses electrons to form cations:
Ca(s) +2H + (aq)
Ca 2+ (aq) + H
2
(g)
– Oxidized: atom, molecule, or ion becomes more positively charged.
• Oxidation is the loss of electrons.
– Reduced: atom, molecule, or ion becomes less positively charged.
• Reduction is the gain of electrons.
Video
31
Introduction to Oxidation-Reduction
Reactions
Figure 4.20:
A summary of an oxidationreduction process, in which M is oxidized and X is reduced.
32
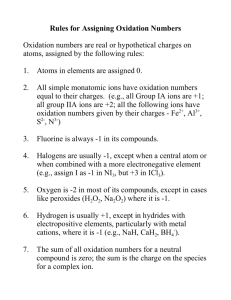
Oxidation Number Rules
Memorize these!!
1. Pure elements have an oxidation state = 0.
2. Monatomic ions have an oxidation state = to their charge.
3. Oxygen has an oxidation state = -2,
(except in peroxides, where it = 1)
4. Hydrogen has an oxidation state of +1
(except when combined with metals, where it = -1.
5. Fluorine is always assigned an oxidation state of -1.
6. The total of the oxidation states of all atoms in a compound must = 0. The total of the oxidation states in an ion must equal the ion charge.
33
Find the oxidation states for each of the elements in each of the following compounds:
• MnO
2
•
PCl
5
• SF
4
• K
2
Cr
2
O
7
•
CO
3
2-
• HClO
4
34
Introduction to Oxidation-Reduction
Reactions
The Half-Reaction Method (pg. 172):
1.
Write separate reduction, oxidation reactions.
2. For each half-reaction:
•
Balance elements (except H,O)
• Balance O using H
2
O
•
Balance H using H +
• Balance charge using electrons
3. If necessary, multiply by integers to equalize # of e .
4. Add half-reactions and eliminate redundancies.
5. Check that elements and charges are balanced.
35
Introduction to Oxidation-Reduction
Reactions
The Half-Reaction Method: (acidic solution)
Turn to pg. 175 and follow the steps.
Sample Exercise 4.19
Potassium dichromate is a bright orange compound that can be reduced to a blue-violet solution of Cr +3 ions. In acidic solutions, K
2
Cr
2
O
7
(C
2
H
5
OH) as follows: reacts with ethyl alcohol
H +
(aq)
+ Cr
2
Cr 3+
(aq)
O
7
2-
(aq)
+ CO
+ C
2(g)
2
H
5
+ H
OH
(l)
2
O
(l)
-->
Balance this equation using the half-reaction method.
36
•
Balance the following oxidation-reduction reactions that occur in acidic solution.
•
ClO-(aq) + I-(aq)
Cl-(aq) + I
3
(aq)
•
Br (aq) + MnO
4
(aq)
Br
2
(l) + Mn 2+ (aq)
•
CH
3
OH(aq) + Cr
2
O
7
2(aq)
CH
2
O(aq) + Cr 3+ (aq)
37
Introduction to Oxidation-Reduction
Reactions
The Half-Reaction Method: (basic solution)
1.
Balance as in acid.
2.
Add OH that equals H + ions (both sides!)
3.
Form water by combining H + , OH -
4.
Check elements and charges for balance.
38
Introduction to Oxidation-Reduction
Reactions
The Half-Reaction Method: (basic solution)
Turn to pg. 178 and follow the steps.
Sample Exercise 4.20
Silver is sometimes found in nature as large nuggets; more often it is found as ores. An aqueous solution containing cyanide ion is often used to extract the silver using the following net reaction that occurs in basic solution:
Ag
(s)
+ CN -
(aq)
+ O
2(g)
--> Ag(CN)
2
-
(aq)
Balance using the half-reaction method.
39
Types of Chemical Reactions and
Solution Stoichiometry
The End
40