Nutrient Role in Bioenergetics
advertisement

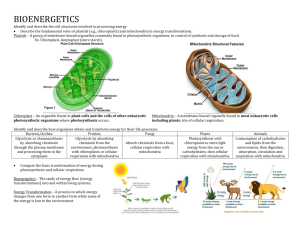
Nutrient Role in Bioenergetics Chapter 4 Bioenergetics Bioenergetics refers to the flow of energy within a living system. Energy is the capacity to do work. Aerobic reactions require oxygen. Anaerobic reactions do not require oxygen. Bioenergetics First law – Energy is neither created nor destroyed, but instead, transforms from one state to another without being used up. Bioenergetics There are six forms of interchangeable energy states: • • • • • • Chemical Light Electric Mechanical Heat Nuclear Bioenergetics The process of photosynthesis is a chemical reaction. Chlorophyll absorbs radiant energy: To synthesize glucose from carbon dioxide and water To release oxygen. Solar energy and photosynthesis provide power to the animal world through food and oxygen. Bioenergetics Photosynthesis What is the equation for the chemical reaction of photosynthesis? Bioenergetics Respiration is the reverse of photosynthesis. C6H12O6 + O2 → 6CO2 + 6H2O Cellular Respiration Organism transforms the chemical energy into a form it can use. Cellular Respiration-Step by step process Glucose Lipids Amino acids Heat Bioenergetics Takes one of three forms: • • • Mechanical work of muscle contraction Chemical work for synthesizing cellular molecules Transport work that concentrates diverse substances in body fluids Bioenergetics Potential energy Kinetic energy Energy associated with a substance’s structure or position. Energy of motion. Potential energy and kinetic energy The total energy of any system. Bioenergetics Adenosine Triphosphate Bioenergetics Cellular Oxidation–Reduction Reactions Constitute the mechanism for energy metabolism Redox reactions power the transfer process of energy Bioenergetics Oxidation–reduction reactions couple: • Oxidation = a substance loses electrons • Transfer oxygen, hydrogen, or electrons Reduction = a substance gains electrons Atoms gain an electron-reducing valence Coupled Reactions Coupled Reactions Reduction Reaction → 2C3H6O3 LDH Pyruvate (gains 2 e-) → Lactate 2C3H4O3 + 2H Coupled Reactions Oxidation Reaction → LDH Lactate (loses 2 e-) 2C3H6O3 - 2H 2C3H4O3 Pyruvate Bioenergetics ATP – energy currency Potential energy extracted from food ATP Chemical energy extracted for biologic work Cells Muscle contraction Phosphate Bond Stored or potential energy High energy bonds ATP – hydrolysis ATP + H2O → ADP + P – 7.3 kCal/mole ATPase Bioenergetics Bioenergetics Phosphocreatine (PC) is also a highenergy phosphate compound. ATP-PC (phosphagens) Releases energy when bonds between creatine and phosphate are broken. Sustains all out exercise ~ 5-8 s Resynthesis of ATP used – reservoir Stored in muscle - anaerobic Bioenergetics Cells store 4-6 times more PCr than ATP Muscle Provide a reservoir of high-energy phosphate bonds ATP + H2O ADP + Pi ATPase ADP + C~P ATP + C Creatine kinase Bioenergetics Phosphorylation Refers to energy transfer through phosphate bonds Oxidative phosphorylation Synthesizes ATP by transfer of electrons NADH and FADH2 Cellular Oxidation-Reduction Reactions Mechanism for energy metabolism Involves transfer of hydrogen atoms Loss of hydrogen: oxidation Gain of hydrogen: reduction Cellular Oxidation Mitochondria NAD and FAD → NADH and FADH2 Cytochromes – Electron Transport Chain (ETC) Transfer of electrons (H+) Energy conserved – high energy phosphate bonds Figure 4.11 Bioenergetics Sources for ATP formation include: • Glucose derived from liver glycogen • Free fatty acids - circulating • Glycogenolysis Triacylglycerol and glycogen stored in muscle Triacylglycerol in liver, adipocytes Lipoprotein complexes - circulating Amino acids Intramuscular and liver-derived carbon skeletons Bioenergetics