Borohydride Reduction of 2-Methylcyclohexanone Lab
advertisement

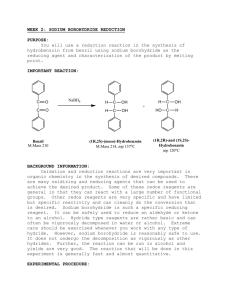
Chapter 26 Borohydride Reduction of 2-Methylcyclohexanone Purpose diastereomers • In this experiment a ketone is reduced with sodium borohydride. • Base is added and the product is extracted to ether. In the usual way this organic layer is dried with sodium sulfate. Removal of the ether leaves the liquid alcohol. Reaction Mechanism • With sodium borohydride, the attacking species is BH4- ion, which, in effect, transfers H- to the carbon. Solvent (MeOH) does participate in the reaction and remains attached to the boron. Comments • Follow pp. 422-423 (microscale). • Follow the lab handout for all experimental procedures. Diethyl ether is to be substituted with methyl t-butyl ether. • Perform IR analysis of the product. • Calculate yield. Low yields are typical. (Moles Product / Moles Starting Material) x 100 = % Yield Safety • Flammable solvents are used in this experiment; keep away from open flame. • Sodium borohydride is toxic by inhalation, in contact with skin and if swallowed and causes burns. Avoid contact with skin and do not inhale dust. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.