Review I
advertisement

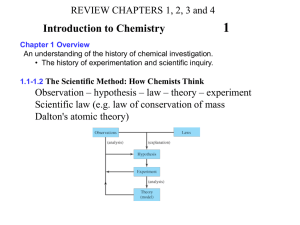
REVIEW CHAPTERS 1, 2, 3 and 10 (part) What is Chemistry Chapter Overview An understanding of the history of chemical investigation. • The history of experimentation and scientific inquiry. •1.1 Science and Technology •1.2 Matter What Is Matter? A. Occupies space and has mass B. Atom – smallest unit of matter C. Molecule – atoms joined together See next slide for classification of matter into Pure substances and mixtures Classifying Matter According to Its State: Solid, Liquid, and Gas A. Solid (fixed volume, incompressible) 1. Crystalline 2. Amorphous B. Liquid 1. Fixed volume 2. Fluid C. Gas (lot if empty space) 1. Compressible 2. Fluid Classifying Matter •1.3, 1.4 and Inserts section 3.5 and 3.6 How We Tell Matter Apart: Physical and Chemical Properties A. Physical property 1. Observable without changing the identity 2. Melting point, odor, color B. Chemical property 1. Observable only by changing the identity-Chemical reactions 2. Flammability How Matter Changes: Physical and Chemical Changes A. Physical change 1. Appearance and properties can change 2. Composition does not change B. Chemical change 1. Appearance and properties can change 2. Composition changes C. Separation of mixtures through physical changes 1. Decanting 2. Distillation 3. Filtration •1.5 The Scientific Method: How Chemists Think Observation – hypothesis – law – theory - experiment Scientific law (e.g. law of conservation of mass) Dalton's atomic theory Numerical Side of Chemistry Chapter Overview A cornerstone of the chemical sciences, the manipulation of numbers and their associated units. Measurement accuracies, significant figures, rounding and scientific notation. • 2.1 and 2.2 Numbers in chemistry–Units and precision and accuracy in reporting it • 2.3 Significant Figures: Writing Numbers to Reflect Position A. How many digits can I report? How many should I report? B. Certain digits and estimated digits C. Counting significant figures 1. All nonzero digits are significant 1234 = 4 Sig fig 2. Interior zeros are significant 505 = 3 sig fig 3. Trailing zeros after a decimal are significant 55.00 = 4 sig fig 4. Leading zeros are not significant 0.012 = 2 sig fig 5. Zeros at the end of a number, without a decimal point, are ambiguous 150 = ambiguous D. Exact numbers • 2.4 Scientific Notation: Writing Big and Small Numbers A. Shorthand notation for numbers B. Two main pieces: decimal and power-of-10 exponent C. Measured value does not change, just how you report it (550.6 to 1 sig fig?) 2.5 Significant Figures in Calculations A. Multiplication and division: Result carries as many significant digits as the factor with the fewest significant digits B. Rounding 1. If leftmost dropped digit is 4 or less, round down (leave it same) 2. If leftmost dropped digit is 5 or higher, round up (increment it by 1) C. Addition and Subtraction Result carries as many decimal places as the quantity with the fewest decimal places D. Calculations Involving Both Multiplication/Division and Addition/Subtraction 1. Do steps in parentheses first 2. Determine the number of significant figures in intermediate answer 3. Do remaining steps 2.6 The Basic Units of Measurement A. English, metric, SI B. SI Units (Mass – kg; Length – m; Time – sec) C. Prefix Multipliers milli (m) 0.001 centi (c) 0.01 kilo (k) 1000 Mega (M) 1,000,000 D. Derived Units 1. Area – cm2 2. Volume – cm3 or L Common Prefixes in the SI System MEMORIZE Prefix Symbol Decimal Equivalent Power of 10 1,000,000 Base x 106 1,000 Base x 103 mega- M kilo- k deci- d 0.1 Base x 10-1 centi- c 0.01 Base x 10-2 milli- m 0.001 Base x 10-3 micro- m or mc 0.000 001 Base x 10-6 nano- n 0.000 000 001 Base x 10-9 2.8 Converting from One Unit to Another (UNIT 1 to UNIT 2) A. Units are important, most numbers get one B. Include units in all calculations C. Conversion factors (Unit you have comes in the bottom, unit you want comes in the TOP) Unit 1 X Unit 2 = UNIT 2 Unit 1 D. Significant figure of the final answer depends on UNIT 1 given in problem NOT the sig. fig of the conversion factor Solving Multistep Conversion Problems A. Understand where you are going first B. Not all calculations can be done in one step 2.8 Units Raised to a Power A. 1 inch = 2.54 cm so 1 inch3 = 2.54 cm3 = 16.4 cm3 2.7 Density (D= Mass/Volume) A. Mass per unit volume (D= Mass/Volume) B. Derived unit (Volume = Mass/Density) (Mass = Density X Volume) C. Can be used as a conversion factor between mass and volume Numerical Problem Solving Strategies and the Solution Map A. Come up with a plan before you pull out your calculator B. Use the units to guide your plan 2.10 Energy A. Energy cannot be created or destroyed B. Units of energy 1. Joule (J) 2. calorie (cal) (1 cal = 4.184J) 3. Calorie (Cal) (1Cal = 1000cal = 1kcal) 4. Kilowatt-hour (kWh)- - Will not be used in CH19 Temperature: Random Molecular and Atomic Motion A. Fahrenheit (F) B. Celsius (C) C. Kelvin (K) Conversions F - 32 C 1.8 And 1.8 C 32 F K C 273 Calorimetry: Measuring Quantities of Heat Read definitions of Specific heat (cal/gºC) meaning of it Specific of heat of water = 1cal/g ºC EXAM 1- 100 POINTS Feb. 21, 2012, 6:00 – 7:30 pm, Room T-109 Multiple Choice Fill in the Blanks scientific notation, chemical change, physical change, chemical and physical properties, Pure substances and mixtures Show calculations for partial/full credit Simple Conversions –show all work round to correct to correct Sig. Fig, scientific notation when indicated Density, calories, temperature Show all calculations/work Simple Calorimetry problems for the first test