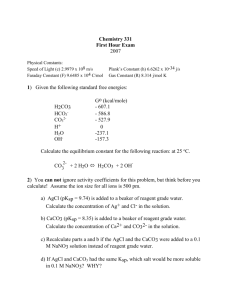
Starter S-30
advertisement

Starter S-105 1. How many grams of copper (II) chloride are in 3.83 x 1021 formula units? Chapter 11 Chemical Reactions Chapter 11 11.1 Describing Chemical Reactions 11.1 Describing Chemical Reactions Writing Chemical Reactions Word Equations – gives the names of compounds and elements in the equation reactants products Reactants – compounds and elements before the reaction Products – compounds and elements after the reaction 11.1 Describing Chemical Reactions Different compounds, or elements are separated by a plus sign Copper + Oxygen Copper (II) Oxide These are often converted from descriptions of the reaction 11.1 Describing Chemical Reactions When antiseptic hydrogen peroxide is put in an open cut bubbles of oxygen form rapidly. Water is also produced. Reaction – reactants hydrogen peroxide 11.1 Describing Chemical Reactions When antiseptic hydrogen peroxide in an open cut bubbles of oxygen from rapidly. Water is also produced. Reaction – products hydrogen peroxide 11.1 Describing Chemical Reactions When antiseptic hydrogen peroxide in an open cut bubbles of oxygen from rapidly. Water is also produced. Reaction – products hydrogen peroxide oxygen + water 11.1 Describing Chemical Reactions When a stove burner is lit, methane gas reacts with oxygen to form carbon dioxide and water. Reaction – reactants methane gas + oxygen 11.1 Describing Chemical Reactions When a stove burner is lit, methane gas reacts with oxygen to carbon dioxide and water. Reaction – products methane gas + oxygen 11.1 Describing Chemical Reactions When a stove burner is lit, methane gas reacts with oxygen to carbon dioxide and water. Reaction – products methane gas + oxygen carbon dioxide + water 11.1 Describing Chemical Reactions Chemical Equations – replace the names of compounds and elements with the chemical formula hydrogen peroxide oxygen + water H 2 O 2 O 2 + H 2O Remember diatomic elements 11.1 Describing Chemical Reactions methane gas + oxygen carbon dioxide + water CH4 + O2 CO2 + H2O 11.1 Describing Chemical Reactions Usually the state of each compound or element is given beside the formula Solid (s) Liquid (l) Gas (g) Aqueous (aq) CH4(g)+ O2(g) CO2(g) + H2O(g) Starter S-106 When solutions of potassium iodide and lead (II) nitrate are mixed in a beaker, a brilliant yellow solid is formed. This solid when analyzed turns out to be lead (II) iodide. Further analysis shows that potassium nitrate is now dissolved in the water. A. Write the word equation. B. Write the chemical equation 11.1 Describing Chemical Reactions Balancing Chemical Equations – both sides of a chemical equation have the same number of each atom Mass is conserved Balancing Demo 11.1 Describing Chemical Reactions Example Al + O2 Al2O3 1. Write elements on each side and how many of each are present 11.1 Describing Chemical Reactions Example Al + Al – 1 O–2 O2 Al2O3 Al – 2 O–3 2. Add one element at a time to the side that doesn’t have enough 11.1 Describing Chemical Reactions Al Al + Al – 2 O–2 O2 Al2O3 Al – 2 O–3 11.1 Describing Chemical Reactions Al Al + Al – 2 O–4 O2 O2 Al2O3 Al – 2 O–3 11.1 Describing Chemical Reactions Al Al + Al – 2 O–4 O2 Al2O3 O2 Al2O3 Al – 4 O–6 11.1 Describing Chemical Reactions Al,Al,Al O2 Al2O3 Al + O2 Al2O3 Al – 4 O–4 Al – 4 O–6 11.1 Describing Chemical Reactions Al,Al,Al O2,O2 Al2O3 Al + O2 Al2O3 Al – 4 Al – 4 O–6 O–6 Balanced 3. Count the number of each compound or element – write a coefficient for each 11.1 Describing Chemical Reactions 4Al + 3O2 2Al2O3 11.1 Describing Chemical Reactions Balance the following equations A. Cu + AgNO3 Cu(NO3)2 + Ag 11.1 Describing Chemical Reactions Balance the following equations A. Cu + 2AgNO3 Cu(NO3)2 + 2Ag B. FeCl3 + NaOH Fe(OH)3 + NaCl 11.1 Describing Chemical Reactions Balance the following equations A. Cu + 2AgNO3 Cu(NO3)2 + 2Ag B. FeCl3 + 3NaOH Fe(OH)3 + 3NaCl C. CS2 + Cl2 CCl4 + S2Cl2 11.1 Describing Chemical Reactions Balance the following equations A. B. C. D. Cu + 2AgNO3 Cu(NO3)2 + 2Ag FeCl3 + 3NaOH Fe(OH)3 + 3NaCl CS2 + 3Cl2 CCl4 + S2Cl2 AgNO3 + H2S Ag2S + HNO3 11.1 Describing Chemical Reactions Balance the following equations A. B. C. D. E. Cu + 2AgNO3 Cu(NO3)2 + 2Ag FeCl3 + 3NaOH Fe(OH)3 + 3NaCl CS2 + 3Cl2 CCl4 + S2Cl2 2AgNO3 + H2S Ag2S + 2HNO3 Zn(OH)2 + H3PO4 Zn3(PO4)2 + H2O 11.1 Describing Chemical Reactions Balance the following equations A. B. C. D. E. F. Cu + 2AgNO3 Cu(NO3)2 + 2Ag FeCl3 + 3NaOH Fe(OH)3 + 3NaCl CS2 + 3Cl2 CCl4 + S2Cl2 2AgNO3 + H2S Ag2S + 2HNO3 3Zn(OH)2 + 2H3PO4 Zn3(PO4)2 + 6H2O Fe2O3 + H2 Fe + H2O 11.1 Describing Chemical Reactions Balance the following equations A. B. C. D. E. F. Cu + 2AgNO3 Cu(NO3)2 + 2Ag FeCl3 + 3NaOH Fe(OH)3 + 3NaCl CS2 + 3Cl2 CCl4 + S2Cl2 2AgNO3 + H2S Ag2S + 2HNO3 3Zn(OH)2 + 2H3PO4 Zn3(PO4)2 + 6H2O Fe2O3 + 3H2 2Fe + 3H2O Starter S-107 1. Balance C6H6 + O2 CO2 + H2O CH3CH2OH + O2 CO2 + H2O Chapter 11 11.2 Types of Chemical Reactions 11.2 Reactions 11.2Type TypesofofChemical Chemical Reactions You will need to know and predict the product for 6 types of chemical reactions 1. Synthesis Form Example A B AB Synthesis 2 H 2 ( g ) O2 ( g ) 2 H 2O( g ) 11.2 Reactions 11.2Type TypesofofChemical Chemical Reactions 2. Decomposition Form AB A B Example 2H 2O( g ) 2H 2( g ) O2( g ) electricity Decomposition 11.2 Reactions 11.2Type TypesofofChemical Chemical Reactions 3. Single Replacement Reaction Form A BC B AC Example Zn( s ) Cu( NO3 ) 2( aq) Cu( s ) Zn( NO3 ) 2( aq) For metals, the reactions occurs if the metal is more reactive than the the one in the compound Single 11.2 Reactions 11.2Type TypesofofChemical Chemical Reactions 4. Double Replacement Reaction Form AB CD AD CB Example Na2 S( aq) Cd ( NO3 )2( aq) CdS( s ) 2 NaNO3( aq) Double 11.2 Reactions 11.2Type TypesofofChemical Chemical Reactions 5. Combustion Reaction Form AB O2 AO BO Example 2C8 H18(l ) 25O2( g ) 16CO2( g ) 18H 2O Combustion 11.2 Reactions 11.2Type TypesofofChemical Chemical Reactions 6. Acid/Base Neutralization Reaction Form HA BOH AB H 2O Example HCl( aq) NaOH ( aq) NaCl( aq) H 2O(l ) The substance AB is called a Chemical Salt Acid Base 11.2 Reactions 11.2Type TypesofofChemical Chemical Reactions Reactions are predicted based on recognizing forms. Starter S-110 What type of reaction is this? CaCl2 + Pb(NO3)2 Write the complete balanced equation 11.2 Reactions 11.2Type TypesofofChemical Chemical Reactions Reaction AgNO3 + NaCl Type: Double Replacement Products: AgNO3 + NaCl AgCl + NaNO3 Balanced: AgNO3 + NaCl AgCl + NaNO3 11.2 Reactions 11.2Type TypesofofChemical Chemical Reactions Reaction Pb + AgNO3 Type: Single Replacement Products: Pb + AgNO3 Ag + Pb(NO3)2 Balanced: Pb + 2AgNO3 2Ag + Pb(NO3)2 11.2 Reactions 11.2Type TypesofofChemical Chemical Reactions Reaction FeCl3 + KOH Type: Double Replacement Products: FeCl3 + KOH Fe(OH)3 + KCl Balanced: FeCl3 + 3KOH Fe(OH)3 + 3KCl 11.2 Reactions 11.2Type TypesofofChemical Chemical Reactions Reaction NaOH + H2SO4 Type: Acid Base Neutralization Products: NaOH + H2SO4 Na2SO4 + H2O Balanced: 2NaOH + H2SO4 Na2SO4 + 2H2O 11.2 Reactions 11.2Type TypesofofChemical Chemical Reactions Reaction C2H8 + O2 Type: Combustion Products: C2H8 + O2 CO2 + H2O Balanced: C2H8 + 4O2 2CO2 + 4H2O 11.2 Reactions 11.2Type TypesofofChemical Chemical Reactions Reaction NH3 Type: Decomposition Products: NH3 N2 + H2 Balanced: 2NH3 N2 + 3H2 11.2 Reactions 11.2Type TypesofofChemical Chemical Reactions Reaction Bi(NO3)3 + H2S Type: Double Replacement Products: Bi(NO3)3 + H2S Bi2S3 + HNO3 Balanced: 2Bi(NO3)3 + 3H2S Bi2S3 + 6HNO3 Starter S-111 What type of reaction is Fe + CuCl2 Write and balance the reaction Chapter 11 11.3 Reactions in Aqueous Solutions 11.2 Type of Chemical Reactions 11.3 Reactions in Aqueous Solution Many reactions take place in water – aqueous For example, the reaction of Silver Nitrate and Sodium Chloride AgNO3( aq) NaCl( aq) AgCl( s ) NaNO3( aq) But in a solution, most ionic compounds have dissociated to form ions 11.2 Type of Chemical Reactions 11.3 Reactions in Aqueous Solution That is they are really in this form Ag ( aq) 3( aq) NO ( aq) Na ( aq) Cl ( aq) AgCl( s ) Na 3( aq) NO This is called the Complete Ionic Equation AgNO–3(the NaCl AgCl(into NaNOonly Notice solid is( aq not broken aq ) ) s ) ions, 3( aq ) what is in solution 11.2 Type of Chemical Reactions 11.3 Reactions in Aqueous Solution Look carefully at the equation Ag ( aq) 3( aq) NO ( aq) Na ( aq) Cl ( aq) AgCl( s ) Na 3( aq) NO There are Na+ and NO3- ions that stay the same These are called Spectator Ions A Net Ionic Equation shows only ions that actually do something in a reaction 11.2 Type of Chemical Reactions 11.3 Reactions in Aqueous Solution Net Ionic Equation Ag ( aq) 3( aq) NO ( aq) Na Ag ( aq) Cl ( aq) AgCl( s ) Na 3( aq) NO Try this one ( aq()s ) 3( aq 3((aq )aq)) 2 ( aq) 3 2( aq) 3( aq) Pb( s ) Pb 2( sAg Pb 2 AgNO 22NO Ag 2 2 Ag Ag Pb Pb ( NO ) 2 NO ) ( s )( s ) 11.2 Type of Chemical Reactions 11.3 Reactions in Aqueous Solution Practice Equation Na3 PO4( aq) FeCl3( aq) 3NaCl( aq) FePO4( s ) Compete Ionic Equations 3Na(aq) PO4(3aq) Fe(aq3 ) 3Cl(aq) 3Na(aq) 3Cl(aq) FePO4( s ) Net Ionic Equation PO4(3aq) Fe(aq3 ) FePO4( s ) Starter S-112 Write the complete ionic and net ionic equations for Fe(NO3)3(aq) + 3NaOH(aq) Fe(OH)3(s) + 3NaNO3(aq) Complete Ionic Fe+3(aq)+3NO3-(aq) + 3Na+(aq)+3OH-(aq) Fe(OH)3(s)+ 3Na+(aq)+3NO3-(aq) Net Ionic Fe+3(aq)+3OH-(aq) Fe(OH)3(s) 11.2 Type of Chemical Reactions 11.3 Reactions in Aqueous Solution Precipitate – separates from a solution as a solid Predicted using a solubility Chart chart In the reaction AgNO3( aq) NaCl( aq) AgCl NaNO3 Is either product a solid? 11.2 Type of Chemical Reactions 11.3 Reactions in Aqueous Solution AgNO3( aq) NaCl( aq) AgCl NaNO3 AgCl – insoluble NaNO3 11.2 Type of Chemical Reactions 11.3 Reactions in Aqueous Solution AgNO3( aq) NaCl( aq) AgCl NaNO3 AgCl – insoluble - solid NaNO3 11.2 Type of Chemical Reactions 11.3 Reactions in Aqueous Solution AgNO3( aq) NaCl( aq) AgCl NaNO3 AgCl – insoluble - solid NaNO3 – soluble 11.2 Type of Chemical Reactions 11.3 Reactions in Aqueous Solution AgNO3( aq) NaCl( aq) AgCl NaNO3 AgCl – insoluble - solid NaNO3 – soluble - aqueous