Osmosis, diffusion and types of solutions
advertisement

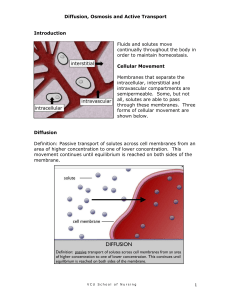
Bell Ringer 1. 2. 3. 4. 5. 12-07-09 What is an example of an acid? What is an example of a base? If a solution has a pH of 2 it is a(an) _____. If a solution has a pH of 7 it is _____. If a solution has a pH of 13 it is a(an) _____. Osmosis, diffusion, and types of solutions Solutions A well mixed mixture that contains a solvent and one or more solutes is a solution. Solvents and solutes _____ are present in the largest amount. They dissolve the other substances. _____ are present in the smallest amount and are dissolved by the solvent. Diffusion Diffusion = the gradual, spreading out of molecules from an area of greater to lesser concentration. Osmosis Osmosis = the diffusion of water through a semi-permeable ( selectively permeable) membrane from an area of greater concentration of water to an area of lesser concentration of water. 3 Types of Solutions Types of solutions 1. Isotonic solution – when the solution outside a cell has the same concentration of solutes and the same concentration of water molecules as the cell content. The cell neither gains nor loses water. 2. Hypertonic- A cell is surrounded by an environment with a higher concentration of solutes than within the cell itself, resulting in water leaving the cell through osmosis. This causes the cell to shrink. 3. Hypotonic- when the cell contains more solute than its surroundings, and water rushes into the cell This causes the cell to swell until it bursts! The opposite of a hypertonic environment is a hypotonic one, Osmotic pressure on blood cells diagram Osmosis Osmosis