CHAPTER 10 AND 11
advertisement

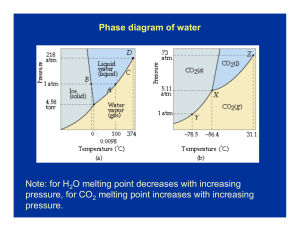
CHAPTER 10 AND 11 AP CHEMISTRY INTERMOLECULAR FORCES Molecules tend to be Nonconductors of electricity when pure substances Most molecules when placed in water won’t conduct – EXCEPTION- highly polar molecules i.e. HCl Insoluble in water but soluble in nonpolar solvents (iodine dissolves in alcohol) i.e. toluene, CCl4 A few are very soluble in water i.e. ethyl alcohol(C2H5OH) Low melting and boiling points Many are gases at room temperature Many boiling points are directly related to the strength of the intermolecular forces DIPOLE FORCES Ion-dipole – Ions attracted to a dipole substance i.e. NaCl in water Dipole-dipole What are dipole forces? – Attraction between polar molecules As the dipole moment increases the boiling point increases London dispersion forces (Van der Waal) What is a dispersion force? – Electrical temporary dipoles (POLARIZABILITY) All molecules have dispersion forces, the strength depend on two factors Number of electrons in the molecule Ease with which electrons are dispersed to form temporary dipoles As the molar mass increases the strength will increase HYDROGEN BONDS Polarity has a small effect on boiling point Compounds have to have hydrogen attached to nitrogen, oxygen or fluorine Hydrogen in one compound is attracted to a lone pair on the N, O, or F of a different compound Two reasons hydrogen bonding is stronger than other dipole forces Electronegative difference is large between hydrogen(2.2) and nitrogen(3.0), oxygen(3.5), or fluorine(4.0) Small size of hydrogen allows the unpaired electron pair of F, O, or N atom to approach the H atom closely The molar mass of H2S is double that of water, both are polar molecules, but the boiling point of water is 167 °C higher than H2S. Why? PROPERTIES OF LIQUIDS Viscosity –Resistance to flow Surface tension –Energy required to increase surface area of liquid by a unit amount –Water increases surface tension because of hydrogen bonding PHASE CHANGES Vapor pressure Pvapor = Patm - PHg column page 460-461 Heat of fusion – Enthalpy change associated with melting Ice = 6.01 kJ/mol Heat of vaporization – Heat required to vaporize a liquid Water = 40.67 kJ/mol CRITICAL TEMPERATURE Highest temperature at which a substance can exist as a liquid Critical pressure – Pressure required to bring about liquidefaction Sublimation – When a substance goes from the solid phase to the gaseous phase without going through the liquid phase Deposition – When a substance goes from the gaseous phase to the solid phase without going through the liquid phase VAPOR PRESSURE Dynamic equilibrium – Opposing processes are occurring at the same rate – Evaporation and condensation Volatile – Liquids with high vapor pressure evaporate quickly Phase diagram – On the overhead STRUCTURE OF SOLIDS Crystalline solid – Geometry shaped to their arrangement of ions Amorphous solid – No orderly arrangement – They do not melt at a specific temperature – Glass Crystal Lattice Unit cell repeated, page 432 CONTINUE Close packing of ions cause three types of unit cells Simple Body centered Face centered Page 447 X-RAY DIFFRACTION Scattering of x-rays by a regular arrangement of atoms or ions Constructive interference – Higher intensity if waves are the same Destructive interference – Cancels each other out Bonding in solids – Table 10.3 page 436 – Graphite and diamonds are allotropes of carbon (same number of carbons (60) but in different forms – You can convert graphite into diamonds Read pages 440 to 470 take notes SOLUTION COMPOSTION Molarity = moles of solute L of solution Percent mass = mass of solute X 100 Mass of solution Mole fraction = A = XA = nA nA + nB nA = moles of solute nB = moles of solution or other substance Molality = mole of solute kg of solvent NORMALITY Equivalents/L solution Acid-base – Equivalent mass of acid or base that can get 1 mole of protons – Equivalent mass of sulfuric acid is ½ it’s molar mass because you get 2 protons Oxidation-reduction – Equivalent amount of oxidizing or reducing agent to accept or furnish 1 mole of electrons – KMnO4 ENERGY SOLUTION FORMATION Enthalpy Hsol’n = H1 + H2 + H3 Fig 11.2 page 490 and table 11.3 page 492 Read and take notes 492-497 Vapor pressure – Nonvolatile solute lowers the vapor pressure of a solvent Raoult’s law Psol’n =Xsolvent·Posolvent – Po = vapor pressure of solvent – X = mole fraction of solvent CONTINUE Ptotal = XAPoA + XBPoB Liquid-liquid solution that obey Raoult’s law is an ideal solution Table 11.4 page 503 COLLIGATIVE PROPERTIES Nonvolatile solute raises the boiling point and lowers the freezing point ΔTf = kfm m = mol solute/kg solvent ΔTb = kbm ΔT = change in temperature kf or kb = boiling point or freezing pt. constant OSMOTIC PRESSURE Semipermeable membrane – Solvents can pass through but not the solute molecule The volume of solution increases and the solvent decreases, this flow is called OSMOSIS Osmotic pressure – When you have an excess pressure on the solution in comparison to the pure solvent CONTINUE Reverse osmosis – If an external pressure greater than the osmotic pressure is applied the solvent particles will go from the solution to the pure solvent Colligative properties of electrolytes Van’t Hoff factor i: i = moles of particles in solution Moles of solute dissolved Colloids – Suspension of tiny particles in some medium – Blood – Coagulation - destroy a colloid - heating or adding an electrolyte (ions)