States of Matter
advertisement

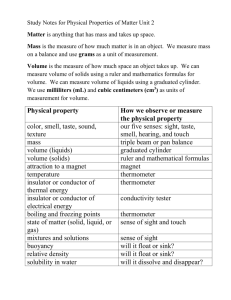
States of Matter The Four States of Matter • • • • Solid Liquid Gas Plasma Solid A fixed, closely packed arrangement of particles causes a solid to have a definite shape and volume Types of Solids • Solids that are made up of crystals are called crystalline solids • In amorphous solids, the particles are not arranged in a regular pattern Crystalline Solids Amorphous Solids Liquid Because its particles are free to move, a liquid has no definite shape. However, it does have a definite volume. Fluid Any substance that can flow Air flows over the wings of an airplane Water flows when poured into a flask Properties of Liquids Surface Tension: An inward pull among molecules of a liquid that brings the molecules on the surfaces closer together Homework Get a paper clip to float on water and take a “Selfie” with it. If you need help refer to your book, Properties of Liquids on pg 46 Viscosity • A liquids resistance to flow • High Viscosity = Flows Slowly Low-------------------High Gases As they move, gas particles spread apart, filling all the space available. Thus, a gas has neither definite shape nor definite volume. Gases Key Terms • Copy the key terms onto flashcards for this section • Obtain a hand mirror, clean it with a dry cloth and describe the mirror’s surface (paragraph pointing out physical properties of the mirror) Mirror Homework Obtain a hand mirror, clean it with a dry cloth and describe the mirror’s surface (paragraph pointing out physical properties of the mirror) Hold the mirror about 15cm away from your face and try to breath against the mirror. Reduce the distance until you can observe a visible change. Describe what you see. (this should be another paragraph) Using the descriptions from the mirror assignment … • What did you observe when you breathed on the mirror held close to your mouth • Explain this observation • Why did you get different results when the mirror was a greater distance from your face? Changes of State Look at H2O Ice Cream Cone 1. Increase thermal energy 2. Particle movement increases 3. Break free of fixed position 4. Ice cream melts Solid to Liquid • The change of state from solid to a liquid is called melting • In most pure substances melting occurs at a certain temperature called the melting point 0°C / 32 °F Liquid to Solid • The change of state from a liquid to a solid is called freezing • At freezing temperature, the particles are moving so slow that they begin to form regular patterns 0°C / 32 °F How to make Ice • Water loses energy to the cold air in the freezer • Particles slow down do to lose of energy • Particles form a regular pattern at 0°C • Temperature remains at 0°C until freezing is complete Liquid to Gas Vaporization happens when the particles in a liquid gain enough energy to form a gas 2 types: Evaporation and Boiling Evaporation • Takes place only on the surface of a liquid • Gains energy from sources like… – Ground – Air – Sun • This added energy allows for surface particles to escape as a gas Boiling • Takes place at surface and below the surface – This is why you see bubbles! • Temperature at which a substance boils is call the boiling point • Boiling point is directly related to air pressure Boiling and Air Pressure There is no air pressure in space, it is a vacuum. What happens to the liquids in your body when you are in space without a space suit? Gas to Liquid • The opposite of vaporization is Condensation • The particles in a gas lose thermal energy to form a liquid That white stuff is liquid water!! You can not see water vapor Solid to Gas • Sublimation occurs when the surface particles of a solid gain enough energy to become a gas The “fog” is water (H2O) The solid is Carbon Dioxide (CO2) Analyzing Data • Complete the analyzing data box on page 52. • Copy the questions and the answers • Copy the key terms on flash cards from this section Gas Behavior