CHOICE OF REACTOR
advertisement
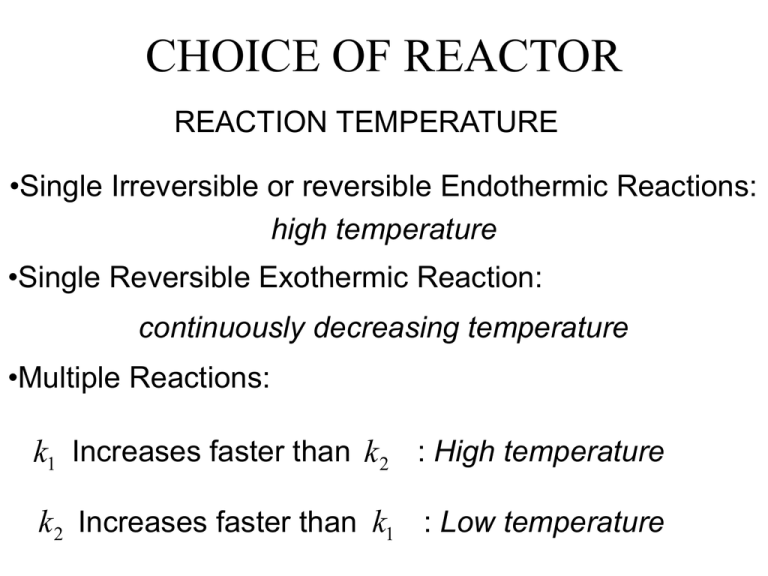
CHOICE OF REACTOR REACTION TEMPERATURE •Single Irreversible or reversible Endothermic Reactions: high temperature •Single Reversible Exothermic Reaction: continuously decreasing temperature •Multiple Reactions: k1 Increases faster than k 2 : High temperature k 2 Increases faster than k1 : Low temperature CHOICE OF REACTOR REACTION TEMPERATURE Temperature Control •Adiabatic Operation: simplest and cheapest •Indirect Heat Transfer: jacket or coil •Cold or Hot Shots: injection of feed at intermediate points. •Heat Barrier: inert material that increases the overall heat capacity flowrate. •Quench: rapid cooling of reactor effluent. CHOICE OF REACTOR REACTION PRESSURE Vapor-phase •Single Irreversible Reactions: as high as practical •Single Reactions with decrease in the number of moles: as high as practical •Single Reactions with increase in the number of moles: continuously decreasing •Multiple Reactions: choose P such Selectivity is maximized CHOICE OF REACTOR REACTION PRESSURE Liquid-phase •Effect of P is less pronounced •P is chosen to: •Prevent vaporization of products •Allow vaporization as a means of removing heat •Allow selective vaporization in a rev. reaction to increase conversion. CHOICE OF REACTOR CATALYSTS •HOMOGENEOUS: reaction occurs entirely in the vapor or liquid phase. •HETEROGENEOUS: catalyst is in a different phase from the reacting species. •Bulk material: a noble metal wire mesh •Supported: active material is dispersed over the surface of a porous media. CHOICE OF REACTOR CATALYSTS DEGRADATION •Deterioration comes with time and produces: •Lower Reaction Rate •Lower Conversion • Can compensate by increasing temperature, but •Can degrade Selectivity •Can accelerate catalyst degradation CHOICE OF REACTOR CATALYSTS DEGRADATION •Physical Loss •Surface Deposits: impede reaction •Sintering: reduce surface area •Poisoning: the poison reacts or bonds with reactant(s) CHOICE OF REACTOR PRACTICAL REACTORS •Stirred-tank Reactors •Tubular Reactors •Fixed-bed Catalytic Reactors •Fixed-bed Non-catalytic Reactors •Fluidized-bed Reactors CHOICE OF REACTOR Stirred-tank Reactors •Batch, semi-batch, or continuous •Batch: •variable production rates •several products in same equipment •Continuous operation: •Automatic Control is much straightforward •Can get closer to IDEAL CSTR is fluid is no too viscous. CHOICE OF REACTOR Stirred-tank Reactors •Batch, semi-batch, or continuous •Batch: •variable production rates •several products in same equipment CHOICE OF REACTOR Stirred-tank Reactors •Continuous operation: •Automatic Control is much straightforward •Can get closer to IDEAL CSTR is fluid is no too viscous. •Not GOOD choice for high pressure. •Not GOOD choice if reactant or products are hazardous or toxic. CHOICE OF REACTOR Tubular Reactors •Bundle of tubes where the movement is in one direction only. •GOOD choice when control of residence time is critical. •GOOD choice for high pressure operation. CHOICE OF REACTOR Fixed-bed Catalytic Reactors •Tubular Reactor packed with catalyst solid particles •Temperature control could be difficult due to variation along the bed. •Exothermic reactions: Local hot points can produce sintering. Use mixed of catalyst and inert solid to dilute heat. CHOICE OF REACTOR Fixed-bed Non-catalytic Reactors •~GOOD for gas-solid reactions. •Non-steady state operation. •Need to be taken off-line for regeneration • GOOD for Gas-liquid •The solid bed provides active area. •Concurrent operation is preferred if short liquid residence time is needed.










