Cell Potential - Dr. Sande's chemistry Courses
advertisement

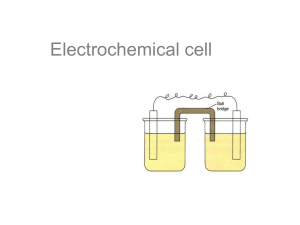
Unit 7: Redox Cell Potentials What is going on in the Copper Lab? What did you observe at the pencil that was connected to the red + terminal? Orange/brown stuff collected on the pencil What did you observe at the pencil that was connected to the black terminal? Bubbles, smell of chlorine. The Electrochemical Cell Source: www.battterybot.com Source: http://www.reuk.co.uk Alkaline Battery Lead-Acid (car) Battery The Electrochemical Cell The species being oxidized LOSES electrons Occurs at the anode (both start with vowels!) Is the reducing agent Is the more active metal Will go from metal to ion, thus putting out electrons The Electrochemical Cell The species being reduced GAINS electrons Occurs at the cathode (both start with consonants!) Is the oxidizing agent Is the less active metal Will go from ion to metal, thus taking in electrons The Electrochemical Cell Cu2+ + Zn → Zn2+ + Cu 1. 2. 3. Zinc Copper 4. 5. ZnSO4 CuSO4 6. Write the oxidation halfreaction Write the reduction halfreaction What is the oxidizing agent? What is the reducing agent? Where is the cathode? Where is the anode? Image: http://commons.wikimedia.org/wiki/File:Galvanic_cell_unlabeled.svg The Electrochemical Cell Cu2+ + Zn → Zn2+ + Cu 1. 2. 3. 4. 5. 6. Write the oxidation halfreaction Write the reduction halfreaction What is the oxidizing agent? What is the reducing agent? Where is the cathode? Where is the anode? The Electrochemical Cell Cu2+ + Zn → Zn2+ + Cu How can we determine the voltage (the cell potential) created by an electrochemical cell? Zinc Copper Electrons flow from anode to cathode. We can measure this. ZnSO4 CuSO4 Image: http://commons.wikimedia.org/wiki/File:Galvanic_cell_unlabeled.svg Determining Cell Potentials 1. Write the oxidation and reduction half-reactions 2. Use the standard reduction table to determine: Ecell = Eoreduction + Eooxidation *Remember to flip the sign if you flip the half-reaction! Cell Potential Example Example: Determine the standard reduction potentials and say if the reaction is spontaneous as written: Mn2+ + 4H2O + Sn2+ → MnO4- + 8H+ + Sn