7th Annual International Diovan Symposium
advertisement

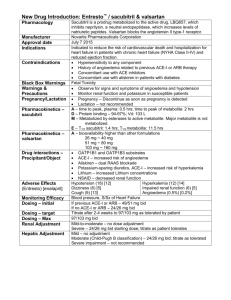
7th Annual International Diovan Symposium Lisbon, 3–5 February 2006 Sponsored by Novartis Pharma AG From the Expert’s Files: Case Presentation Victor Dzau Duke University, Durham, USA Sponsored by Novartis Pharma AG Presentation 52-year-old African-American woman Museum curator History of – Type II diabetes (diet controlled) – Retinopathy and nephropathy Referred to specialist due to BP = 160/100 mmHg despite amlodipine 10 mg, bendrofluazide 2.5 mg and atenolol 50 mg Examination Not overweight Questioning reveals – ex-smoker for 5 years having smoked 20 cigarettes a day from age 16 years – some breathlessness on exertion Clinic BP = 164/103 mmHg Pulse regular Auscultation: – Abdominal bruit – AF Investigations Creatinine = 250 μmol/L (2.82 mg/dL) Mid-stream urine (MSU) = 2+ protein Sugar = 9 mmol/L (162 mg/dL) HbA1C = 7% (normal <5%) Total cholesterol = 5 mmol/L (193 mg/dL) Chest x-ray = normal ECG = sinus rhythm, LVH on voltage criteria Echo = EF 55%, LVH 7th Annual International Diovan Symposium Lisbon, 3–5 February 2006 VARIABLE 3: Hypertension and Microalbuminuria Sponsored by Novartis Pharma AG Pathophysiology of Microalbuminuria in Hypertension Michel Burnier CHUV, Lausanne, Switzerland Sponsored by Novartis Pharma AG Definition of Microalbuminuria 24-hour urines Urine spot mg/24 hours µg/min mg/L mg/mmol creatinine <30 <20 <20 <2 Microalbuminuria 30–300 20–200 20–200 2–20 Macroalbuminuria >300 >200 >200 >20 Category Normal Natural History of Diabetic Nephropathy Clinical type 2 diabetes Functional changes* Structural changes† Rising blood pressure Microalbuminuria Proteinuria Rising serum creatinine levels End-stage renal disease Cardiovascular death Onset of diabetes 2 5 10 20 Years *Renal haemodynamics altered, glomerular hyperfiltration †Glomerular basement membrane thickening , mesangial expansion , microvascular changes +/- 30 Pathophysiological Processes Leading to Albuminuria and Glomerular Lesions Glucose Urinary protein Glycoxidation (glycation) AGEs = angiotensin AT1 receptor Increased glomerular pressure Ang II Efferent arteriolar constriction Ang II Albuminuria and Progression of Nephropathies Glomerular permeability for macromolecules Excessive reabsorption of proteins in the proximal tubule Intracellular accumulation of protein degradation products Gene activations chemokines and cytokines Proliferation of fibroblasts and extracellular matrix Development of fibrosis and renal atrophy Remuzzi et al. Kidney Int 1997;51:2–15 Prevalence of Microalbuminuria in Patients with Hypertension* 40 38.0 33.7 Prevalence (%) 30.0 30 19.8 20 10 6.7 6.1 4.1 0 Bigazzi 1992 Calvino 1999 Grandi Pontremoli Palatini 2000 1997 1996 Jensen 1997 *Defined as > 140/90 mmHg except Calvino, Palatini (135/85 mmHg) Jensen (> 140/90 mmHg or on AHY) Diercks et al. Can J Cardiol 2002;18:525–35 Mean Microalbuminuria is Associated with Left Ventricular Hypertrophy and Carotid Hypertrophy in Hypertensive Patients Left ventricular mass index 200 Intima/media thickness * *** IMT (mm) LVMI (g/m2) ** 0.8 150 100 50 0 * **** 1 0.6 0.4 0.2 C Ht AI– Ht AI+ 0 C C = control; Ht = hypertensive; Al– = no albuminuria; Al+ = with albuminuria *p<0.001 intergroup comparison; **p<0.001 compared to C; ***p<0.05 compared to Ht Al–; ****p<0.01 compared to Ht Al– Pontremoli et al. Am J Hypertens 1998;11:430–8 Ht AI– Ht AI+ Microalbuminuria as a Predictor of Vascular Disease in Non-diabetic Subjects Odds ratio for coronary heart disease Age (10 years) Male sex Systolic BP Diastolic BP Body mass index (10 kg/m2) Current or ex-smoker Treatment of hypertension Diabetes or insulin resistance Microalbuminuria 1 5 10 Odds ratio Yudkin et al. Lancet 1988;2:530–3 15 20 Microalbuminuria and Risk of CV Events, CHF and Death in the HOPE Trial 4 MI/Stroke/CV death All-cause mortality CHF hospitalisation Relative risk 3 2 1 0 <0.22 0.22–0.57 0.58–1.62 Alb/Crea (mg/mmol) Adjusted for age, sex, SBP/DBP, waist-hip ratio, diabetes and HbA1c Gerstein et al. JAMA 2001;286:421–6 >1.62 Renal Insufficiency, Albuminuria and CV Survival in the HOPE Trial HR for primary outcome (CV death, MI, stroke) 2.5 Systolic and diastolic BP NOT significant risk factors 2.0 1.5 1.0 0.5 0 S. creat >124 µmol/L Microalbuminuria Mann et al. Ann Intern Med 2001;134:629–36 Both Albuminuria and CV Diseases LIFE study, 8,029 subjects with hypertension and LV hypertrophy, mean age 66 years Prevalence (%) 40 Normoalbuminuria Microalbuminuria (Alb/Crea >3.5 mg/mmol) Macroalbuminuria (Alb/Crea >35 mg/mmol) 30 20 10 0 Diabetes Cerebrovascular disease Peripheral vascular disease Wachtell et al. J Hypertens 2002;20:405–12 Coronary vascular disease Endpoint rate (%) Composite Endpoints (CV Death, Non-fatal Stroke and MI) Stratified by Time-varying Albuminuria in the LIFE Trial >3 mg/mmoL (n=2,435, 1,708, 1,760) 1–3 mg/mmoL (n=2,219, 1,827, 1,946) 0.5–1 mg/mmoL (n=1,591, 1,587, 1,814) 0.5 mg/mmoL (n=1,961, 3,385, 2,458) 24 22 20 18 16 14 12 10 8 6 4 2 0 0 6 12 18 24 30 36 42 Month Ibsen et al. Hypertension 2005;45:198–202 48 54 60 66 Microalbuminuria and Mortality in the General Population: the PREVEND Study n=85,421 subjects, age: 28–75 years from the Groningen area Non-CV death 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 0.5 0 Hazard ratio Hazard ratio CV death 1 10 100 1,000 Urinary albumin concentration (mg/L) 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 0.5 0 1 10 1,00 1000 Urinary albumin concentration (mg/L) Hillege et al. Circulation 2002;106:1777–82 Microalbuminuria and CV Complications in Hypertension: Is the Threshold Correct? The Copenhagen City Heart Study Cox-estimated age-adjusted curves of cumulative incidence of coronary heart disease for a 60-year-old person based on 1,734 hypertensive subjects with microalbuminuria and normoalbuminuria RR of CHD UAE 4.8 µg/min UAE <4.8 µg/min 30 3 2 1 0 20 <2.5 2.5–5 5–10 >10 4 10 0 0 2 4 6 8 Years from entry 10 12 Klausen et al. Hypertension 2005;46:33–7 RR of death Cumulative mortality (%) 4 3 2 1 0 <2.5 2.5–5 5–10 >10 UAE (µg/min) Microalbuminuria and Incidence of CV Events: The Framingham Study Survival free of CVD According to sex-specific median UACR Percentage 100 95 < Median Median 90 0 1 2 3 4 Years Arnlov et al. Circulation 2005;112:969–75 5 6 7 8 What Links Microalbuminuria to CV Risk ? Microalbuminuria 24-hour Blood Pressure Profile in Clinically Healthy Subjects With or Without Microalbuminuria Microalbuminuria (n=26) Normoalbuminuria (n=45) Blood pressure (mmHg) 165 140 115 90 65 40 0 0 4 8 12 Clock time Clausen et al. Hypertension 1998;32:71–7 16 20 24 Expression of Endothelial Dysfunction in Humans Endothelial dysfunction Impaired endothelium-dependent vasodilation Reduces vasodilation Increased endothelin Favours vasoconstriction Increased transcapillary escape rate of albumin Increases permeability (microalbuminaria) Increased von Willebrand factor Increases prothrombotic activity Increased tPA and PAI-1 Reduces profibrinolytic activity Increased E-selectin and VCAM-1 Leucocytes adhesion and permeability Increased ICAM-1 Induces inflammation Increased fibronectin and type IV collagen fragments Alters matrix synthesis Flow-associated Vasodilation of Brachial Artery in Clinically Healthy Subjects According to Microalbuminuria Flow-associated dilatation (%) p<0.05 105 104 103 102 101 100 0 Normoalbuminuria Elevated UAE Clausen et al. Circulation 2001;103:1869–74 Pathobiological Processes Potentially Involved in the Development and Progression of Vascular Diseases Dyslipidaemia Hypertension Diabetes Smoking Oxidative stress Endothelial dysfunction NO, local mediators, RAAS (Ang II) Vasoconstriction Thrombosis Inflammation Plaque rupture Adapted from Dzau. Hypertension 2001;37:1047–52 Vascular lesion and remodelling Chronic Kidney Disease and CV Risk Traditional risk factors Non-traditional risk factors Age Albuminuria Sex Homocysteine Hypertension LP(a) and apolipoproteins HDL and LDL cholesterol Anaemia Diabetes Ca/phosphate metabolism Smoking Salt and water overload Physical activity Oxidative stress Family history of CVD Inflammation LVH Malnutrition Thrombogenic factors Sleep disturbance NO/endothelin balance… Vascular Effects of Angiotensin II Vasoconstriction Stimulation of Ang II type 1 receptors Release of endothelin and norepinephrine Reduction of NO bioactivity and production of peroxynitrite Inflammation Activation NADH/NADPH oxidase and production of superoxide anion Induction of MCP-1, VCAM, TNF-a, IL-6 expression Activation of monocytes and macrophages Remodelling Stimulation of SMC migration, hypertrophy and replication Induction of PDGF, bFGF, IGF-1, TGF-b expression Stimulation of matrix glycoproteins and metalloproteinase expression Thrombosis Stimulation of PAI-1 synthesis and change in tPA/PAI-1 ratio Activation of platelet with increased aggregation and adhesion Angiotensin II Inhibition Retards the Progression of Renal Diseases Prevention Benedict Study Normoalbuminuria Protection IRMA 2 IDNT MARVAL RENAAL Microalbuminuria Macroalbuminuria ESRD CV morbidity and mortality Early stage Late stage Terminal stage Severity of renal disease Reduction in Albuminuria Translates Into a Decrease in CV Events in Hypertensive Patients: LIFE Study Fraction suffering composite endpoint 0.20 High baseline/high year 1 High baseline/low year 1 Low baseline/high year 1 Low baseline/low year 1 0.15 0.10 0.05 0 0 10 20 30 40 50 Follow-up (months) Ibsen et al. Hypertension 2005;45:198–202 60 70 Effect of Fosinopril on CV Event Rates in Patients with Microalbuminuria 1.00 Event-free survival 0.98 0.96 0.94 0.92 HR 0.60 [0.33–1.10], p=0.098 (Log-rank) 0.90 Placebo Fosinopril 0.10 0 0 10 20 30 Follow-up (months) Asselbergs et al. Circulation 2004;110:2809–16 40 Event-free Survival According to the Level of Microalbuminuria 1.00 Event-free survival 0.95 0.90 0.85 p=0.008 UAE UAE UAE UAE 0.80 0.10 <50 mg/24 hours, placebo >50 mg/24 hours, placebo <50 mg/24 hours, fosinopril >50 mg/24 hours, fosinopril 0 0 10 20 30 Follow-up (months) Asselbergs et al. Circulation 2004;110:2809–16 40 Conclusions Microalbuminuria is frequent in hypertension and is associated with target organ damage and the incidence of CV complications The pathophysiological link between microalbuminuria and CV risk is not completely understood but it may be due to endothelial dysfunction with an impaired NO balance, activation of local mediators and increased activity of the RAAS system Blockade of the RAAS with ACE inhibitors or AT1 receptor blockers is an important therapeutic approach to reduce microalbuminuria and to prevent the development of CV and renal complications in hypertension 7th Annual International Diovan Symposium Lisbon, 3–5 February 2006 Sponsored by Novartis Pharma AG Point-Counterpoint Are Benefits Beyond Blood Pressure Lowering Clinically Relevant? Sponsored by Novartis Pharma AG Albuminuria-associated Disease: Are Benefits Beyond BP Lowering Clinically Relevant? Giancarlo Viberti, MD Professor of Diabetes and Metabolic Medicine Cardiovascular Division KCL School of Medicine Guy’s Hospital King’s College London London, UK Age-specific Relation of Usual BP to Vascular Mortality In Individuals With No Previous Vascular Disease Prospective Studies Collaboration. Lancet 2002;360:1903–13 Annual Transition Rates Through Stages of Diabetic Nephropathy No nephropathy 2.0% (1.9% to 2.2%) 1.4% (1.3% to 1.5%) Microalbuminuria 2.8% (2.5% to 3.2%) 3.0% (2.6% to 3.4%) Macroalbuminuria 2.3% (1.5% to 3.0%) Elevated plasma creatinine or renal replacement therapy 4.6% (3.6% to 5.7%) 19.2% (14.0% to 24.4%) Adler et al. Kidney Int 2003;63:225–32 Relationship Between SBP and ACR in T2DM Patients with Different Degrees of AER ACR mg/mmol 100 10 1 0.1 80 100 120 140 160 180 200 220 240 SBP mmHg Smith et al. JASN 2005;16:1069–75 Risk factors for microalbuminuria in type 1 diabetic patients with baseline normoalbuminuria (7 yr follow-up) Excess Mortality With Hypertension and Proteinuria In Type 2 Diabetes Status of hypertension (H) and proteinuria (P) in type 2 diabetes 1000 Standardised mortality ratio 500 0 P-H- P-H+ P+H- P+H+ P-H- P-H+ P+H- P+H+ Men Women Wang et al. Diabetes Care 1996;19:305–12 Epidemiology Relative Risk of Cardiovascular Disease and Mortality in Diabetes Mellitus By Quartile of Albuminuria (ACR) ACR (mg/mmol) quartiles RR (95% CI) 1st 2nd 3rd 4th <0.22 0.22–0.57 0.58–1.62 >1.62 p for trend MI, stroke and CV death 1 0.85 1.11 1.89 <0.001 (0.63–1.14) (0.86–1.43) (1.52–2.63) All-cause mortality 1 0.86 1.41 2.38 (0.58–1.28) (1.01–1.95) (1.80–3.20) CHF 1 0.72 1.83 3.65 (0.32–1.63) (0.98–3.43) (2.06–6.46) Variable <0.001 <0.001 n=3,498 Gerstein et al. JAMA 2001;286:421–6 Rate of eGFR Decline in Type 2 DM With Normoalbuminuria AER categories: I = ≤10 mg/24h II = 10.1 to 20 mg/24h III = 20.1 to 30 mg/24h Rachmani et al. Diabetes Res Clin Pract 2000;49:187–94 Survival Curves in Type 2 DM According To Baseline AER Category AER categories: I = ≤10 mg/24h II = 10.1 to 20 mg/24h III = 20.1 to 30 mg/24h Rachmani et al. Diabetes Res Clin Pract 2000;49:187–94 Albuminuria and CVD risk in hypertensive patients with LVH The LIFE Study ACR (mg/mmol) <0.25 ≥0.25 to <0.82 ≥0.82 to<1.62 ≥1.67 to<4.32 ≥4.32 to <9.45 P value for trend HR 1 1.3 1.8 2.3 2.7 <0.001 Adjusted HR 1 1.3 1.5 1.9 2 <0.001 Composite endpoint Composite endpoint = CVD death, fatal or non-fatal stroke, fatal or non-fatal MI Relative Risk of CVD and Mortality in 5,545 High-risk Patients Without Diabetes by Quartile of Albuminuria (ACR) ACR (mg/mmol) quartiles RR (95% CI) Variable 1st 2nd 3rd 4th <0.22 0.22–0.57 0.58–1.62 >1.62 p for trend MI, stroke and CV death 1 1.24 1.54 1.83 (1.03–1.49) (1.29–1.85) (1.52–2.20) <0.001 All-cause mortality 1 1.17 1.49 2.27 (0.93–1.47) (1.19–1.87) (1.82–2.82) <0.001 CHF 1 1.45 1.86 2.93 (0.87–2.44) (1.12–3.10) (1.79–4.81) <0.001 Gerstein et al. JAMA 2001;286:421–26 Albuminuria and Incidence of CVD Events in Non-hypertensive and Non-diabetic Subjects The Framingham Heart Study Survival free of CVD According to sex-specific median UACR Median UAER: M: 3.9 μg/mg F: 7.5 μg/mg Arnlov et al. Circulation 2005;112:969–75 Albuminuria and Risk of CHD and Death In The General Population Third Copenhagen City Heart Study 25%-ile: 2.1 μg/min 50%-ile: 3.0 μg/min 75%-ile: 4.8 μg/min Klausen et al. Circulation 2004;110:32–35 Albuminuria and CVD/Non-CVD Mortality in The General Population PREVEND Study Hillege et al. Circulation 2002;106:1777–82 The Clinical Trial Evidence Change in AER Predicts Loss of GFR Rossing et al. Diabetologia 1994;37:511–16 RENAAL: Change From Baseline in Proteinuria* 40 Placebo 20 Median percent change 0 p=0.0001 35% overall reduction -20 -40 Losartan -60 0 P (+CT) 762 L (+CT) 751 12 24 Months 36 48 632 661 529 558 390 438 130 167 *Proteinuria measured as the urine albumin: creatinine ratio from a first morning void Brenner et al. N Engl J Med 2001;345:861–9 RENAAL: Baseline Proteinuria As A Determinant of Renal Events In T2DM Composite Endpoint 100 3.0 g/24h 100 80 60 <1.5 g/24h 40 20 0 % with ESRD endpoint % with renal endpoint 80 ESRD 3.0 g/24h 60 40 20 <1.5 g/24h 0 0 12 24 Month 36 48 0 12 24 36 48 Month De Zeeuw et al. Kidney Int 2004;65:2309–20 RENAAL: Baseline Proteinuria As A Determinant For Cardiac Events In T2DM CV Endpoint ≥3.0g/24h % with CV endpoint Heart Failure 60 40 <1.5 g/24h 20 0 % with heart failure endpoint 60 40 3.0 g/24h 20 <1.5 g/24h 0 0 12 24 Month 36 48 0 12 24 36 48 Month De Zeeuw et al. Circulation 2004;110:921–7 RENAAL: Initial Antiproteinuric Response vs Renal Risk ESRD Hazard ratio Renal Endpoint 2.5 2.5 2.0 2.0 1.5 1.5 1.0 1.0 0.5 0.5 0.0 0.0 -90 -25 0 25 50 72 Albuminuria reduction (%) -90 -25 0 25 50 72 Albuminuria reduction (%) De Zeeuw et al. Kidney Int 2004;65:2309–20 RENAAL: Proteinuria Reduction (<0% versus >30%) Determines the Cardiovascular Outcome % with CV endpoint <0% >30% 30 20 10 Heart Failure 40 % with heart failure CV Endpoint 40 30 20 <0% 10 >30% 0 0 0 12 24 Month 36 48 0 12 24 36 48 Month De Zeeuw et al. Circulation 2004;110:921–7 Changes In BP and AER By Valsartan and Amlodipine in T2DM Patients With Microalbuminuria The MARVAL Study UAER (µg/min) Mean BP change (mmHg) SBP DBP 70 0 2 60 4 40 6 30 50 8 -6.6 10 12 p <0.001 -6.5 20 10 -11.2 -11.6 0 Valsartan Amlodipine Baseline Valsartan 24 Wks Amlodipine 24 Wks Viberti et al. Circulation 2002;106:672–8 Arterial blood pressure (mmHg) Blood Pressure According To Treatment Group 160 Systolic 150 140 130 120 Trandolapril Verapamil Trandolapril plus Verapamil Placebo 110 100 90 Diastolic 80 70 0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 48 Follow-up (months) Ruggenenti et al. N Engl J Med 2004 20 No ACE inhibitor Cumulative incidence of microalbuminuria (%) (66 events) 15 10 5 ACE inhibitor (35 events) A.F. (95 % C.I.) = 0.44 (0.27 – 0.70) p=0.001 0 0 6 12 18 24 30 36 42 48 Follow-up (months) No. at risk ACE inhibitor 601 503 469 441 417 399 380 311 220 No ACE inhibitor 603 463 424 405 376 357 338 270 188 Ruggenenti et al. N Engl J Med 2004 Rate of CVD Events By Time-varying Albuminuria In Subjects With Essential Hypertension and LVH The LIFE Study Composite endpoint CV death, fatal or non-fatal stroke, fatal or non-fatal MI Ibsen et al. Hypertension 2005;45:198–202 Risk of ESRD vs Initial Change( 6–0 months) in Proteinuria in African Americans with Hypertension and Non-diabetic Kidney Disease AASK Lea et al. Arch Intern Med 2005;165:947–953 How do we obtain better evidence? Antihypertensive and Antiproteinuric Responses To Increasing ACE-I Dose Lisinopril dose (mg) 5 mg 10 mg 15 mg 20 mg 0 -10 -20 -30 % reduction vs control -40 -50 -60 -70 BP Urinary protein -80 Adapted from Palla et al. Int J Clin Pharmacol Res 1994;14:35–43 Conclusions Albuminuria is a powerful and independent risk factor for renal and cardiovascular disease. The relationship is linear across a range which includes normalcy Correction of albuminuria per se appears to be related to reduction of risk of renal and cardiovascular events To acquire direct clinical evidence a trial is required that compares different doses of the same compound with similar BP-lowering effects but different albuminuria reduction potency Are Benefits Beyond BP Lowering Clinically Relevant? No Giuseppe Mancia University of Milan-Bicocca, Italy Sponsored by Novartis Pharma AG The Question Does BP reduction per se substantially contribute to CV protection (i.e. reduction in CV morbidity and mortality) in hypertension? Effects of Antihypertensive Drugs on CVD in Controlled Trials CVD (%) Comparator Diuretics –16 Placebo Beta-blockers –21 Placebo Calcium antagonists –28 Placebo ACE inhibitors –24 Placebo Ang II antagonists –10 Active therapy* * BP –2/–1 mmHg Metanalysis of Trials Comparing Different Treatments or Treatment Versus Placebo in Hypertension 1.50 Stroke 1.50 Major CVD 1.25 1.25 1.25 1.00 1.00 1.00 0.75 0.75 0.75 0.50 0.50 0.50 0.25 –10 –8 –6 –4 –2 0 1.50 Relative risk of outcome event Relative risk of outcome event 1.50 2 0.25 4 –10 –8 –6 –4 –2 0 1.50 CVD death 2 CHD 0.25 4 –10 –8 –6 –4 –2 0 Total mortality 1.25 1.25 1.00 1.00 0.75 0.75 0.50 0.50 0.25 –10 –8 –6 –4 –2 0 0.25 –10 –8 –6 –4 –2 0 2 4 SBP difference between randomised groups (mmHg) Turnbull et al. Lancet 2003;362:1527–35 2 4 2 4 VALUE: Analysis of Results Based on BP Control at 6 Months Patients treated with valsartan Patients treated with amlodipine Odds ratio Fatal/non-fatal cardiac events Fatal/non-fatal stroke * * All-cause death * 0.50 (0.39–0.64) * * 0.79 (0.69–0.91) 0.62 (0.50–0.77) 0.4 0.6 0.8 1.0 1.2 Controlled Non-controlled patients† patients (n=5,253) (n=2,396) Hazard ratio 95% CI *p<0.01; †SBP <140 mmHg at 6 months Weber et al. Lancet 2004;363:2047–49 0.73 (0.63–0.85) * 0.79 (0.69–0.92) 0.91 (0.71–1.17) 0.83 (0.66–1.03) Myocardial infarction Heart failure hospitalisations 0.76 (0.66–0.88) 0.60 (0.48–0.74) * Odds ratio * 0.64 (0.52–0.79) 0.4 0.6 0.8 1.0 1.2 Controlled Non-controlled patients† patients (n=5,502) (n=2,094) Hazard ratio 95% CI FEVER: Endpoint Analysis (First Time Occurrence in Each Category) Per 1,000 patient-years Felodipine Placebo Hazard ratio (95% CI) (138.1/82.3 mmHg) (141.6/83.9 mmHg) Stroke 11.2 Fatal 2.1 Non-fatal 9.1 All CV events 15.2 All cardiac events 4.6 All-cause death 7.1 CV death 4.6 Coronary events 4.5 Heart failure 1.1 New-onset diabetes 3.1 Cancer 2.6 15.9 3.1 12.7 21.2 6.6 9.6 6.4 6.2 1.7 2.7 3.9 0.72 0.70 0.72 0.72 0.66 0.70 0.68 0.68 0.76 1.03 0.60 0.4 0.6 0.81.0 1.5 2.0 Felodipine better Liu Lisheng et al. J Hypertens 2005 Placebo better More Versus Less Intensive Treatment in DM + DM + (n=3,599) BP –6.0/–4.6 mmHg 0 Stroke CHD CHF CVD Total CV death mortality Risk ratio –10 –16 –20 –25* –30 –40 –31 –36* *Statistically significant Turnbull et al. Arch Intern Med 2005;165:1410–19 –27* –33 Clinical Outcomes – Unadjusted BP control by visit Percent of visits with BP control (<140/90 mmHg) HR Primary outcome <25% 1.00 25%–<50% 0.67 50%–<75% 0.60 75% 0.54 Reduced risk (0.59–0.76) (0.53–0.67) (0.48–0.61) MI (fatal + non-fatal) <25% 25%–<50% 50%–<75% 75% 1.00 0.70 0.63 0.55 (0.57–0.86) (0.53–0.76) (0.46–0.65) Stroke (fatal + non-fatal) <25% 25%–<50% 50%–<75% 75% 1.00 0.88 0.62 0.43 (0.66–1.18) (0.47–0.82) (0.32–0.58) Increased risk (95% CI) 0.40 0.60 0.80 HR (95% CI) 1.00 Group with <25% of visits with BP control used as reference Primary Outcome = first occurrence of death (all cause), non-fatal MI, or non-fatal stroke 1.20 Does CV protection (reduction in CV morbidity and mortality) exclusively depend on BP reduction per se? Are there specific protective effects of different drugs or drug classes? CV Events in Patient Subgroups 2.9/1.7 mmHg Amlodipine/perindopril (BP 164.1/94.8 135.5/79.1 mmHg) Atenolol/thiazide (BP 163.9/94.5 136.3/78.4 mmHg) Diabetes No diabetes Current smoker Non-current smoker Obese Non-obese LVH No LVH Older (>60 years) Younger (≤60 years) Female Male Previous vascular disease No previous vascular disease Renal dysfunction No renal dysfunction With metabolic syndrome Without metabolic syndrome 0.70 0.80 0.90 1.00 1.50 Major CVD with ACE-I Versus D/BB CA Favours first BP (mmHg) Favours second Versus RR (95% CI) I2 (%) ACE-I versus D/BB Diabetes –0.5/0.1 No diabetes 0.6/0.1 Overall 0.90 (0.74–1.11) 55 1.04 (0.98–1.10) 0 p homog = 0.19 CA versus D/BB Diabetes 0.7/–0.6 No diabetes 1.4/–0.2 Overall 0.95 (0.82–1.10) 1.04 (0.98–1.10) p homog = 0.82 0 0 ACE-I versus CA Diabetes 0.4/1.2 No diabetes 0.4/0.8 Overall 0.92 (0.79–1.07) 0.99 (0.92–1.07) p homog = 0.37 0 0 0.25 0.5 1 Risk ratio Turnbull et al. Arch Intern Med 2005;165:1410–19 2 Meta-analysis of Trials Comparing ACE-I-based with ARB-based Regimens for the Outcomes of Stroke, CHD and Heart Failure Outcome Trial Favours ARB Stroke ELITE II OPTIMAAL VALIANT Overall 1.63 (0.77–3.44) 1.06 (0.84–1.33) 0.95 (0.76–1.17) 1.02 (0.87–1.19) Major CHD ELITE II OPTIMAAL VALIANT Overall 1.24 (1.00–1.55) 1.01 (0.88–1.15) 0.97 (0.89–1.05) 1.03 (0.92–1.16) Heart failure ELITE II OPTIMAAL VALIANT Overall 0.87 (0.59–1.28) 1.14 (0.99–1.31) 1.01 (0.93–1.11) 1.05 (0.95–1.15) 0.5 Favours ACE-I 1.0 Relative risk Relative risk (95% CI) 1.5 Blood Pressure Lowering Treatment Trialists’ Collaboration Relative Risk of MI for ARBs and ACE-Is Versus Active Drugs and Placebo ARBs versus ACE-I ARBs versus placebo and active drug ARBs versus active drug ARBs versus placebo 0.4 0.5 0.6 0.7 0.8 0.9 1.0 Favours ARB Volpe et al. J Hypertens 2005;23:2113–18 1.1 1.2 1.3 Favours other drug 1.4 Should Guidelines Convey the Message that What Matters for CV Protection is Only BP Control? CVD by many drugs (and drug combinations), provided BP For a given BP little/no CVD between treatments Benefit proportional to degree of BP BP control versus lack of control associated with large CVD Tighter BP control (well below 140/90 mmHg) associated with greater CV protection (high-risk patients) Short-term Protection BP reduction Drug Mancia, 2004 May Event-based Trials Underestimate Potential Differences Between Drugs? Trial limitations – High-risk patients – Patients’ drop-out/cross-over (dilution factor) – Short-term duration Pseudoequivalence? Prevention of events not superimposable to prevention of disease Differences Between Drugs on Factors Responsible for Progression of Disease BP lowering Probably not Lipid profile Yes, minor Insulin resistance Yes New-onset diabetes Yes Metabolic syndrome Yes LVH progression/regression Yes Small vessel remodelling Yes Large artery structure/function/atherosclerosis Yes Renal protection Yes Role of Drug-specific Properties Versus BP Reduction per se in CV Protection of Hypertensive Patients Short-term protection BP reduction Drug Mancia, 2004 Long-term protection Drug BP reduction ? Effect of Antihypertensive Treatment (n=10) GFR (ml/min/1.73 m2) MAP (mmHg) 125 115 Start of treatment 105 95 105 95 85 75 65 Albuminuria (g/min) 1,250 750 250 –30 –24 –18 –12 –6 0 6 Months Parving et al. Lancet 1983;2:1175–9 12 18 24 30 36 7th Annual International Diovan Symposium Lisbon, 3–5 February 2006 Sponsored by Novartis Pharma AG From the Expert’s Files: Case Presentation Marc Pfeffer Harvard Medical School, USA Sponsored by Novartis Pharma AG Presentation 60-year-old Turkish male lawyer presents for routine check-up History of ischaemic heart disease and hypertension Myocardial infarction 3 years previously, uncomplicated recovery Progressive shortness of breath on exertion for past 3 weeks Current meds – – – – ASA statin beta-blocker ACE-I Examination BP = 110/70 mmHg Height = 1.85 Weight = 93 kg – BMI = 27 Heart rate = 76 No peripheral oedema JVP elevated at 30° Carotid upstrokes normal, no bruit Lungs: basal crepitations Systolic murmur, no S3 Investigations Dipstick protein –ve Creatinine = 141 mmol/L (1.5 mg/dL) eGFR = 52 ECG = Evidence of old anterior MI I aVR V1 V4 II aVL V2 V5 III aVF V3 V6 IV II V5 Echo = Ejection fraction 35%; dilated left ventricle The Multiplicative Effect of Global Risk Factors in Post-MI HF Patients: The Root Cause Peter Liu University of Toronto, Canada Sponsored by Novartis Pharma AG Incidence of Post-MI HF Based on the 44-year follow-up of the NHLBI’s Framingham Heart Study… The incidence of HF approaches 10 per 1,000 population after age 65 Approximately 22% of male and 46% of female MI patients will experience HF within 6 years NHLBI = National Heart, Lung, and Blood Institute Hurst. The Heart, Arteries and Veins. 10th ed. New York, NY: McGraw-Hill, 2001; American Heart Association. Heart Disease and Stroke Statistics – 2005 Update. Dallas, Texas: American Heart Association, 2004 VALIANT Registry: In-hospital Clinical Events Among Post-MI Patients With and Without HF/LVSD 25 HF/LVSD (n=2,347) No HF/LVSD (n=3,219) Patients (%) 20 16 15 13 10 8 7.1 5 2.3 2.5 6 2.2 1.4 0.9 0 Death Reinfarction AF Stroke LOS (days) LVSD = left ventricular systolic dysfunction; AF = atrial fibrillation LOS = length of stay Velazquez et al. Eur Heart J 2004;25:1911–9 Beta-blocker: Carvedilol Post-MI Reduces Cardiovascular Mortality Proportion Event-free 1.00 0.90 Carvedilol n=975 0.80 Placebo n=984 Risk reduction: 25% (4%, 42%) p=0.024 0.70 0.60 Cardiovascular mortality rates: placebo 14%; carvedilol 11% 0 0 0.5 1.0 1.5 2.0 Time (years) Adapted from The CAPRICORN Investigators. Lancet 2001;357:1385–90 Antiplatelet Therapy: Clopidogrel and Aspirin Reduce Risk of Death, MI or Stroke at One Year Aspirin/clopidogrel Placebo Death, MI, or stroke (%) 12 10 8 RRR 26.9% p=0.02 11.5 RRR 19.7% p=NS 6.9 6 5.5 RRR 37.4% p=0.04 4 8.5 4.6 2.9 2 0 Day 28 Day 29 to 1 year Composite RRR = Relative risk reduction; NS = non significant Adapted from Steinhubl et al. for the CREDO Investigators. JAMA 2002;288:2411–20 Statin: Fluvastatin Significantly Reduces the Risk of Cardiac Events After A First Successful PCI In patients with average cholesterol levels, fluvastatin significantly reduced the risk of MACE by 22% (p=0.0127) Patients free from MACE (%) 100 90 Fluvastatin (80 mg/d, n=844) 80 Risk reduction = 22% Placebo (n=833) 70 0 0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 Time post-randomisation (years) PCI = percutaneous coronary intervention; MACE = Major Adverse Cardiac Events Serruys et al. JAMA 2002;287:3215–19 Remodelling Post MI: Renin–Angiotensin Activation Initial infarct Infarct expansion (hours to days) Global remodelling (days to months) Modified from Jessup and Brozena. New Engl J Med 2003;348:2007–18 Acute Ischemia Chronic Repair Mechanical stress Oxydative stress Hypoxia ECM Initial cytokine release Osteopontin TIMPs, MMPs Ischemic Myocytes Cytokine AII, OFR Cytokine Angiotensin Apoptotic Myocytes Hypertrophied Myocytes Angiogenesis VEGF, Angiopoietins Intergin b3 Necrotic Myocytes = Neutrophils = Monocyte = Macrophages = Mast Cells = Collagen = Angiogenesis Nian et al. Circ Res 2004;94:1543–53 Myocyte Stretch and AII Production Angiotensin II Myocyte Transillum’n Leri. J Clin Invest 1998;101:1326–42 Inflammatory Cytokine Levels in Post-MI Patients With and Without HF/Death TNF- a Inflammatory cytokine levels p<0.01 IL- 6 p<0.01 80 p<0.0001 p<0.0001 70 60 50 40 30 20 10 0 Group 1 (n=140) Group 2 (n=44) Group 1 (n=141) Intrahospital Group 2 (n=30) Follow-up Group 1: made up of patients free from death and HF; Group 2: patients with HF and/or death Valgimigli et al. Circulation 2005;111:863–70 Apoptosis in the Failing Human Heart Propidium Iodide Number of labelled myocyte Nuclei/106 Nuclei Deoxyuridine triphosphate labelling 5,000 4,000 3,000 Deoxyuridine Triphosphate 2,000 20 10 0 Control Ischaemic Idiopathic CM DCM CM = cardiomyopathy; DCM = dilated cardiomyopathy Olivetti et al. N Engl J Med 1997;336:1131–41 Matrix Metalloproteinase (MMP) post MI MTMMP/ADAMs uPA/Plasmin IL-1, CD40 Zn TNF, EMPRINN TIMP3 OFR, Chymase ACE / AII Sun et al. Circulation 2004;110:3221–8; Kassiri et al. Circulation Research 2005;97:380–90 MMP Inhibitors ACEi/ARBs Pathophysiology of Ventricular Remodelling in Post-MI HF Increased levels of inflammatory cytokines Changes in the extracellular matrix: increased fibroblast and myocardial matrix metalloproteinase (collagenase) activity Myocyte apoptosis or necrosis Hypertrophy of remaining myocytes Localised ACE/Chymase Presence Post MI Control 3 days 7 days 28 days Renin–Angiotensin Aldosterone System (RAAS) Non-ACE pathways (e.g. chymase) Vasoconstriction Cell growth Na/H2O retention Sympathetic activation Angiotensinogen Renin Angiotensin I AT1 receptor Angiotensin II ACE Cough, angio-oedema benefits? Bradykinin Aldosterone AT2 receptor Inactive fragments ACE = angiotensin-converting enzyme; AT1 = angiotensin II type 1; AT2 = angiotensin II type 2 Vasodilation Antiproliferation (kinins) Post-MI Remodelling: Ang II Modulation by RAAS Blockade Control Ang II modulation Effects of ACE Inhibitor Treatment on All-Cause Mortality Post-MI AIRE SAVE Radionuclide EF ≤ 40% Probability of event 0.40 TRACE Clinical and/or radiographic signs of HF Echocardiographic EF ≤ 35% Placebo 0.35 ACE inhibitor 0.30 0.25 0.20 Placebo: 866/2971 (29.1%) 0.15 ACE inhibitor: 702/2995 (23.4%) 0.10 OR: 0.74 (0.66–0.83) 0.05 0.0 0 Years ACE inhibitor 2,995 Placebo 2,971 1 2,250 2,184 2 1,617 1,521 EF = ejection fraction; OR = odds ratio Flather et al. Lancet 2000;355:1575–81 3 892 853 4 223 138 VALIANT: Valsartan Shows Non-inferiority to ACE Inhibitors Hazard ratio for death from any cause SAVE TRACE Valsartan preserves 99.6% of the mortality benefit of captopril AIRE SAVE, TRACE and AIRE combined VALIANT (Imputed placebo) 0.5 1 Favours active drug Pfeffer et al. N Engl J Med 2003;349:1893–906 2 Favours placebo Summary Patient post-MI with LV dysfunction is at very high risk for deaths, arrhythmias and recurrent events Pathophysiology of ventricular remodelling in post-MI HF – Inflammatory cytokines – Myocyte apoptosis – Hypertrophy of remaining myocytes and hyperplasia of fibroblasts RAAS activation post-MI contributes to adverse ventricular remodelling and mortality Deleterious effects of angiotensin II mediated via AT1 receptor Standard post-MI therapy should include a platelet inhibitor, beta-blocker, statin, and an ACE-I/ARB RAAS Blockade in Post-MI HF and Chronic HF: What’s the Evidence for This Treatment Strategy? Eric J Velazquez Duke University, Durham, USA Sponsored by Novartis Pharma AG The Cardiovascular Continuum Oxidative stress/ endothelial dysfunction Target organ damage Tissue injury (MI, stroke) Atherosclerosis and LVH Pathologic remodelling Target organ dysfunction (HF, renal) Vascular dysfunction Risk factors: diabetes, hypertension AT1 receptor Endstage organ failure Death LVH = left ventricular hypertrophy MI = myocardial infarction; HF = heart failure Adapted from Dzau and Braunwald. Am Heart J 1991;121:1244–63 The Scope of CHD and MI Worldwide, 17 million people die of CVD every year1 More than 60% of the global burden of CHD occurs in developing countries1 It is estimated that in 2005, 1.2 million Americans will have a new or recurrent coronary attack*2 In 2002, nearly 180,000 people died of an MI2 *Coronary attack=definite or probable MI, or fatal CHD CHD=coronary heart disease 1http//www.who.int/cardiovascular_diseases 2American Heart Association. 2005 Heart and Stroke Statistical Update. 2004 MI and CAD: Secondary Prevention Treatment objectives Prevent reinfarction Prevent LV remodelling Prevent progression to HF Reduce risk of arrhythmias Improve survival How can we help these high-risk patients? Treatment of Post-MI Patients with LVSD/Acute HF LVSD or Acute HF Severity of LV damage Antiplatelet + Statin LVSD and Acute HF Death and Major CV Events SAVE AIRE TRACE Radionuclide EF 40% Clinical and/or radiographic signs of HF Echocardiographic EF 35% 0.75* (0.67–0.83) Events (%) 40 ACE-I (n=2,995) Placebo (n=2,971) 30 0.73* 20 (0.63 – 0.85) 0.80* (0.69 – 0.95) 10 0 n= 355 n= 460 Readmission for HF n= 324 n= 391 Reinfarction *Odds ratio (95% CI) Flather et al. Lancet 2000;355:1575–81 n= 1,049 n= 1244 Death/MI or Readmission for HF Early Treatment of Post-MI Patients with LVSD/Acute HF LVSD or Acute HF Severity of LV damage Proven ACE-I Antiplatelet + + Statin SAVE/AIRE/TRACE LVSD and Acute HF Potential Pharmacological Benefits of AT1-receptor Blockade Versus ACE Inhibition AT1-R antagonists ACE inhibitors Chymase Ang II Plasma Ang II (–) (–) Plasma Ang II (+) (–) Bradykinin (–) Prostagladins AT2 NO AT1 AT1 AT2 Cardioprotection Vasodilation Negative chronotropism Anti-fibrosis Anti-growth NO Cardioprotection Vasodilation Cardioprotective effects via angiotensin II binding to AT2 receptor 1,2 Angiotensin II generated by non-ACE dependent pathways also blocked from binding to the AT1 receptor Reduced side-effect profile (ACE inhibition: increased bradykinin = cough) Adapted from Matsubara. Circ Res 1998;83:1182–91 1Unger. JRAAS 2001;2(suppl 2):S4–S7 2Petrie et al. J Am Coll Cardiol 2001;37:1056–61 HF Patients Not Receiving an ACE-I Data from the SPICE registry; N=9,580 9% Intolerant 2% High risk 3% New diagnosis 80% On ACE-I 5% Unable to determine 1% Data missing “Despite the proven benefits of ACE inhibitors, the reported prevalence of ACE inhibitor use among heart failure patients varies from 17% to 86%.” Bart BA et al. Eur Heart J 1999 OPTIMAAL: All-cause Mortality 25 Losartan (n=499 events) Captopril (n=447 events) Event rate (%) 20 15 10 5 Relative risk = 1.13 (0.99–1.28); p=0.069 0 Month Losartan Captopril 0 2,744 2,733 6 2,504 2,534 12 2,432 2,463 Dickstein et al. Lancet 2002 18 2,390 2,423 24 2,344 2,374 30 2,301 2,329 36 1,285 1,309 Early Treatment of Post-MI Patients with LVSD/Acute HF LVSD or Acute HF Severity of LV damage Antiplatelet + Statin + Proven ACE-I or Losartan 50 mg qd SAVE/AIRE/TRACE OPTIMAAL LVSD and Acute HF VALIANT Acute MI (0.5–10 days)—SAVE, AIRE, or TRACE eligible (either clinical/radiological signs of HF or LV systolic dysfunction) Major exclusion criteria – Serum creatinine >2.5 mg/dL – BP <100 mmHg – Prior intolerance of an ARB or ACE-I – Non-consent Double-blind, active-controlled Captopril 50 mg tid (n=4,909) Valsartan 160 mg bid (n=4,909) Captopril 50 mg tid + Valsartan 80 mg bid (n=4,885) Median duration: 24.7 months Event-driven Primary endpoint: Secondary endpoints: Other endpoints: All-cause mortality CV Death, MI, or HF Safety and tolerability Pfeffer et al. Am Heart J 2000;140:727–50 VALIANT: All-cause Mortality Probability of event 0.30 Captopril Valsartan Valsartan + captopril 0.25 0.20 0.15 0.10 0.05 Valsartan vs captopril: HR=1.00; p=0.982 Valsartan + captopril vs captopril: HR=0.98; p=0.726 0 0 6 12 18 Time (months) 24 30 36 No. at risk Captopril 4,909 4,428 4,241 4,018 2,635 1,432 364 Valsartan 4,909 4,464 4,272 4,007 2,648 1,437 357 Valsartan+Captopril 4,885 4,414 4,265 3,994 2,648 1,435 382 HR = hazard ratio Pfeffer et al. N Engl J Med 2003;349:1893–906 VALIANT: Valsartan is Effective at Reducing Cardiovascular Morbidity and Mortality Hazard ratio (97.5% CI) p value CV death (1,657 events) 0.62 CV death or MI (2,234 events) 0.25 CV death or HF (2,661 events) 0.51 CV death, MI, or HF (3,096 events) 0.20 0.8 1 Favours valsartan 1.2 Favours captopril Pfeffer et al. N Engl J Med 2003;349:1893–906 The Effect of Valsartan, Captopril or Both on Atherosclerotic Events After Acute MI: An Analysis of VALIANT Patients with at least one event (%) 25 Captopril (n=4,909) Valsartan (n=4,909) 20 Valsartan + captopril (n=4,885) 15 10 5 0 Myocardial infraction Angina Revascularisation Adapted from McMurray et al. Presented at ESC 2005 Stroke Study Drug Discontinuation 0.4 Captopril Probability of event Valsartan Valsartan + Captopril 0.3 All 0.2 Due to * Adverse * Events 0.1 0 0 6 12 18 Months *p<0.05 vs captopril 24 30 36 Early Treatment of AMI Patients with LVSD/Acute HF LVSD or Acute HF Severity of LV damage Antiplatelet + Statin + Proven ACE-I (Captopril) or Valsartan 160 mg bid or Captopril + Valsartan SAVE/AIRE/TRACE VALIANT LVSD and Acute HF CAPRICORN Survival 1.00 Proportion Event-Free Carvedilol Placebo 0.95 0.90 0.85 0.80 0.75 p=0.031 OR 0.77 (0.60–0.98) 0.70 0 0.5 1 1.5 Years The CAPRICORN Investigators. Lancet. 2001 2 2.5 EPHESUS All-cause Mortality 10 Cumulative incidence (%) 9 8 7 6 5 4 3 Placebo 2 Eplerenone RR = 0.79 (95% CI, 0.64–0.97) p=0.03 1 0 0 3 6 9 12 15 18 21 24 27 Months since randomisation 30 33 36 Eplerenone 3,319 3,044 2,463 1,260 336 0 0 Placebo 3,313 2,983 2,418 1,213 323 2 0 Pitt et al. for EPHESUS Investigators. N Engl J Med 2003;348:1309–21 Early Treatment of AMI Patients with LVSD/Acute HF LVSD or Acute HF LVSD and Acute HF Severity of LV damage Antiplatelet + Statin + Proven ACE-I or valsartan 160 mg bid + Eplerenone 25–50 mg qd Carvedilol SAVE/AIRE/TRACE VALIANT CAPRICORN EPHESUS Cardiac Events Following High-risk MI: The VALIANT Experience Cumulative incidence 0.50 Any CV event 0.40 0.30 Death Heart failure 0.20 Recurrent MI Sudden death or cardiac arrest 0.10 0.00 0 YEAR 1 YEAR 2 YEAR 3 YEAR 4 The Framingham Heart Study: 1987 Cumulative probability of event Risk of heart failure after MI (Age 35 to 94 at diagnosis) 0.5 MI male MI female Matched male Matched female 0.4 0.3 0.2 0.1 0 0 2 4 6 8 10 12 14 16 18 20 Years following MI Cupples et al. The Framingham Study, NIH Publication No. 87–2703. 1987 Baseline BNP and NE and All-cause Mortality BNP NE Survival probability 1.0 1.0 % BNP Mortality (pg/mL) 0.9 9.7 <41 0.8 14.3 41–97 0.7 20.7 98–238 0.6 % NE Mortality (pg/mL) 0.9 0.8 13.8 0.7 16.5 274–394 23.0 395–572 >572 24.2 0.6 32.4 0.5 >238 0.5 0 10 20 30 40 0 Time Since Randomization, months Anand IS. Circulation 2003;107:1278−83 10 20 30 40 Time Since Randomization, months <274 Kaplan-Meier Analysis of Cumulative Rates of Survival in Patients with Heart Failure Chronically Treated With ACE Inhibitor Stratified By Plasma Angiotensin II Levels 1.0 Normal Ang II 0.8 Ang II >16 pg.mL–1 0.6 0.4 0.2 p=0.0002 0 0 2 4 6 Months Roig et al. Eur Heart J 2000;21:53–7 8 10 12 Val-HeFT: Study Design 5,010 heart failure patients ≥ 18 years old; NYHA II–IV; EF <40 %; LVIDd >2.9 cm/m2 of BSA Receiving standard therapy ACE inhibitors (93%), diuretics (86%), digoxin (67%), beta-blockers (35%) Randomized to Valsartan 40 mg bid titrated to 160 mg bid Placebo 906 deaths (events recorded) Primary endpoint was all-cause mortality and the combined endpoint of all-cause mortality and heart failure morbidity LVIDd = left ventricular internal diastolic diameter; BSA = body surface area Cohn J et al. Eur J Heart Fail 2000;2:439–46 Probability of event-free survival Val-HeFT: Valsartan Significantly Reduces Combined Endpoint of Mortality and Morbidity in Overall Population 100 95 90 85 80 Valsartan (n=2,511) 13.2% 75 Risk reduction** 70 Placebo (n=2,499) 65 0 0 3 6 9 12 15 18 Time (months) 21 24 27 Combined endpoint of mortality and morbidity: All-cause mortality, cardiac arrest with resuscitation, hospitalisation for worsening heart failure, or therapy with IV inotropes or vasodilators; **p=0.009 Cohn et al. N Engl J Med 2001;345:1667–75 Probability of event-free survival Val-HeFT: Valsartan Significantly Reduces Heart Failure-related Hospitalisations 100 95 90 85 Valsartan (n=2,511) 80 27.5% 75 Risk reduction** 70 Placebo (n=2,499) 65 0 0 3 6 9 12 15 18 Time (months) First hospitalisation **p<0.001 Cohn et al. N Engl J Med 2001;345:1667–75 21 24 27 Val-HeFT: Reduction in Combined Morbidity/Mortality* and Mortality With Valsartan (No ACE-I Subgroup) Mortality Combined morbidity/mortality Placebo (n=181) 100 100 90 80 70 60 44.0% risk reduction 50 Proportion survived (%) Probability of event-free survival Valsartan (n=185) 90 80 70 33.1% risk reduction 60 p=0.0171 p=0.0002 40 50 0 3 6 9 12 15 18 21 24 27 30 Time since randomisation (month) 0 3 6 9 12 15 18 21 24 27 30 Time since randomisation (month) *First morbid event, including death or hospitalisation Adapted from Maggioni et al. J Am Coll Cardiol 2002;40:1414–21 CHARM-Added: CV Death or CHF Hospitalization 50 538 (42%) 40 Placebo 483 (38%) % 30 Candesartan 20 HR 0.85 (95% CI 0.75–0.96), p=0.011 Adjusted HR 0.85, p=0.010 10 0 0 Number at risk Candesartan 1,276 Placebo 1,272 1 1,176 1,136 2 Years 1,063 1,013 3 3.5 948 457 906 422 McMurray et al. Lancet 2003;362:767–71 NNT = 14 Val-HeFT: Change in Plasma BNP and NE Over Time Valsartan Placebo n=844 40 n=1,710 0 p<0.001 n=1,886 n=1,696 n=829 30 n=1,890 p<0.001 p<0.001 n=823 –20 NE* (pg/mL) Plasma BNP * (pg/mL) 20 p=0.005 p=0.001 p<0.001 n=1,835 n=1,605 n=800 20 10 n=1,633 0 n=1,850 –40 0 4 12 Time (months) –10 24 0 4 12 Time (months) *Mean ± SEM Latini et al. J Card Fail 2001;7(Suppl 2):Abstract 198l Anand et al. Circulation 2001;104(suppl II):Abstract 2813 24 Conclusions RAAS activation contributes to the chain of events (atherosclerosis, LVH) leading to coronary artery disease Elevated RAAS activity is observed in post-MI HF and chronic HF Potential pharmacological benefits of AT1-receptor blockade versus ACE inhibition In high-risk post-MI patients, valsartan is as effective as captopril in reducing the risk of all-cause mortality, CV death, non-fatal MI or hospitalisation for HF Valsartan reduces combined morbidity and mortality in patients receiving prescribed therapy for chronic HF, predominantly because of a reduction in HF hospitalisations