Chapter 9 /10 Test
advertisement

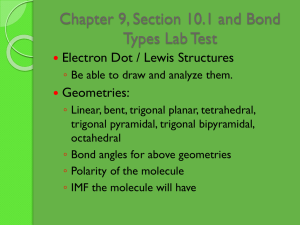
Chapter 9, Section 10.1 and Bond Types Lab Test Electron Dot / Lewis Structures ◦ Be able to draw and analyze them. Geometries: ◦ Linear, bent, trigonal planar, tetrahedral, trigonal pyramidal, trigonal bipyramidal, octahedral ◦ Bond angles for above geometries ◦ Polarity of the molecule ◦ IMF the molecule will have Chapter 9, Section 10.1 and Bond Types Lab Test Hybridization ◦ Hybrid orbitals involving s and p ◦ Sigma and pi bonds ◦ Orbital overlap Molecular orbital diagrams ◦ Realize that this is another method for visualizing molecules ◦ Uses orbital notation similar to what is used for atoms Would either KBr or C7H8 be soluble in water? Use intermolecular forces to explain. ◦ Would either be soluble in hexanes? How do IMFs relate to the following: ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ Viscosity Vapor pressure Boiling point Freezing point State at room temperature Solubility of a substance The bond types lab that we did Intramolecular bonds What is wrong with the localized electron model? What other model improves upon it? What is delocalization? How can we show this is a molecule like benzene? Or NO3-? What is PES? What can we learn from PES? Website with some PES practice: http://www.chem.arizona.edu/chemt/Flash/ph otoelectron.html Use this spectra for the questions in the next slide (these are the types of questions on PES you may have to answer on the test) a. b. c. d. e. f. Assuming that the PES data shows ALL of the electrons present in the atom, identify the element. Still applying the assumption in a., which specific electrons are associated with the peak at 126 eV? What does a relatively low value of energy tell you about the relative position of the electrons within any atom? Which peak in the spectrum represents electrons that are closest to the nucleus? What is the relevance of the relative height of the peaks at 5.31 eV and 9.07 eV? Why is there such a large difference in energy between the peaks at 0.74, 5.31 and 9.07 eV, and the peak at 126 eV? More PES Practice http://www.adriandingleschemistrypages.c om/ap/pes-a-first-attempt-at-creating-aquestion/ http://apchemresources2014.weebly.com/ uploads/9/7/6/4/9764824/photoelectron_s pectroscopy_info.pdf Sections 10.8-10.9 Use the worksheet on heating curves and phase diagrams to help you study. There will be a minimal amount of information on the test over this. Concepts from the Bond Types Lab: ◦ How to determine the type of bond present in a compound. ◦ Properties that each type of compound has including solubility, melting point, appearance, conductivity, etc. What is the chemical reason behind these properties? ◦ What role to IMFs play in this? Can you determine the type of IMF based on properties? ◦ Lab techniques AP Multiple Choice Review From chapter 9: All From chapter 10: 1-7, 10-13, 16-20 Using principles of chemical bonding and/or intermolecular forces, explain each of the following. (a) Xenon has a higher boiling point than neon has. (b) Solid copper is an excellent conductor of electricity, but solid copper chloride is not. (c) SiO2 melts at a very high temperature, while CO2 is a gas at room temperature, even though Si and C are in the same chemical family. (d) Molecules of NF3 are polar, but those of BF3 are not. Explain each of the following in terms of atomic and molecular structures and/or intermolecular forces. (a) Solid K conducts an electric current, whereas solid KNO3 does not. (b) SbCl3 has measurable dipole moment, whereas SbCl5 does not. (c)The normal boiling point of CCl4 is 77ºC, whereas that of CBr4 is 190ºC. (d) NaI(s) is very soluble in water, whereas I2(s) has a solubility of only 0.03 gram per 100 grams of water. Which of the following substances has the greatest solubility in C5H12(l) at 1 atm? (A) SiO2(s) (B) NaCl(s) (C) H2O(l) (D) CCl4(l) (E) NH3(g)