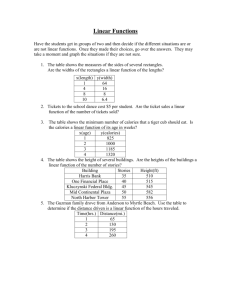
review for quiz - Duluth High School
advertisement

REVIEW FOR QUIZ HEAT • Heat is measured in ____ and is represented with the symbol ___. • Temperature is measured in ___. • Mass is measured in ____. • Specific heat is measured in ___ and is represented with the symbol ____. • • • • Joules, Q Degrees Celsius Grams J/g°C, c • You eat a snack that is 20 g carbs, 4 g fat and 1 g protein. Give the total calories for each macromolecule and then give the total calories for the snack. • • • • 20 x 4 = 80 Calories 4 x 9 = 36 Calories 1 x 4 = 4 Calories Total = 120 Calories 1000 calories = 1 kCal = 1 Calorie 1 calorie= 4.184 Joules Convert the following: • 120 J = ___ calories • 250 Calories = ____ energy calories 120 J 1 cal_ = 28.7 cal 4.184 J 250 C 1000 cal = 250,000 cal 1C • How many joules of energy are absorbed by 250g of water if its temperature rises from 30.0°C to 95.0°C? • Q= mcΔT • Q = (250g)(4.184J/g°C)(65°C) • Q= 67,990 J • Convert this answer into nutritional calories (c) • 67,990J 1cal 1C____ 16.25 C 4.184J 1000cal • How many Joules of energy are released if a block of metal that weighs 250 g with a specific heat of 0.234 J/g°C is cooled from 400 °C to 285°C ? Q= mcΔT Q = (250 J)(0.234J/g°C)(115°C) Q = 6,727.5 J • What is the specific heat of a 50 g piece of metal that takes 300 J of energy to raise the temperature by 40°C? • Q= mcΔT • 300 J = (50 g)(c)(40°C) • c = 0.15 J/g°C • You place a 450 g block of metal in a beaker until the metal reaches a temperature of 85°C. You place the metal in a 250 mL of water in another beaker and measure the temperature to be 30.0°C. When the metal is placed into the water, the final temperature of the system reaches 37.5°C. What is the specific heat of the metal? • mcΔT = mcΔT (450g)(c)(47.5°C) = (250g)(4.184J/g°C) (7.5°C) • 0.42J/g°C