Review of Grade 11
advertisement

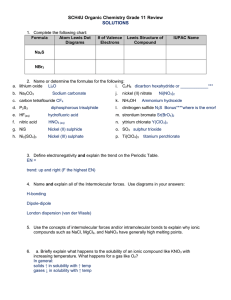
SCH4U Organic Chemistry Grade 11 Review 1. Complete the following chart: Formula Atom Lewis Dot Diagrams # of Valence Electrons Lewis Structure of Compound IUPAC Name Na2S NBr3 a. b. c. d. e. f. g. h. 2. Name or determine the formulas for the following: lithium oxide _________________________ i. C2H6 ____________________________ Na2CO3 _________________________ j. nickel (II) nitrate ___ _______________________ carbon tetraflouride ______________________ k. NH4OH _______________________________ P2S3 _________________________ l. dinitrogen sulfide _________________________ HF(aq) __________________________ m. strontium bromate ________________________ nitric acid __________________________ n. yttrium chlorate ___________________________ NiS ___________________________ o. SO3 __________________________________ Ni2(SO4)3 ___________________________ p. Ti(ClO4)3 ______________________________ 3. Define electronegativity and explain the trend on the Periodic Table. 4. Name and explain all of the Intermolecular forces. Use diagrams in your answers: 5. Use the concepts of intermolecular forces and/or intramolecular bonds to explain why ionic compounds such as NaCl, MgCl2, and NaNO3 have generally high melting points. 6. a. Briefly explain what happens to the solubility of an ionic compound like KNO3 with increasing temperature. What happens for a gas like O2? 7. Write the balanced equation, total ionic equation, and net ionic equation for the reaction between aqueous sodium phosphate and aqueous calcium chloride. ____________________________________________________________________________ ____________________________________________________________________________ ____________________________________________________________________________ 8. Briefly explain what you are looking for in order to classify the following reactions. Synthesis Decomposition Single Displacement Double Displacement Combustion of a Hydrocarbon 9. Four factors affect the rate of a chemical reaction. Identify each factor and explain how/why they each specifically affect the rate of a reaction. 10. Develop the complete combustion reaction for butane. (C4H10). b. Butane is the fuel source in lighters. The combustion of butane is rarely a complete reaction. Explain this observation, and identify two other potential products for this reaction.