SDC 3. Adverse Events and Grade 3 to 4 Laboratory Abnormalities
advertisement
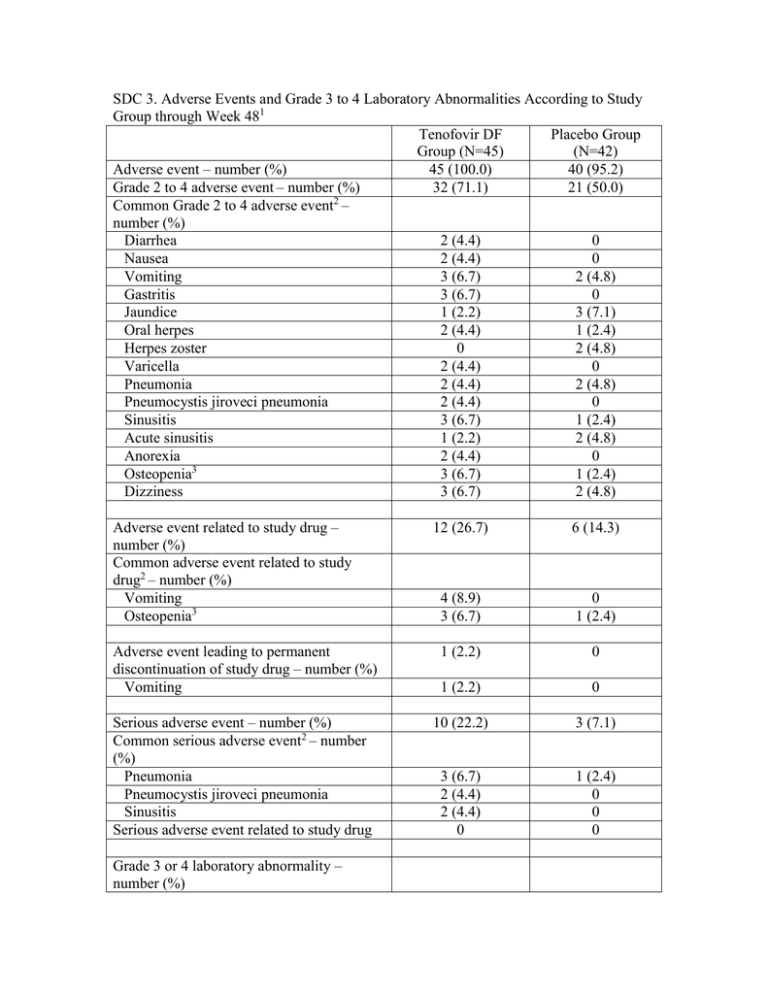
SDC 3. Adverse Events and Grade 3 to 4 Laboratory Abnormalities According to Study Group through Week 481 Tenofovir DF Placebo Group Group (N=45) (N=42) Adverse event – number (%) 45 (100.0) 40 (95.2) Grade 2 to 4 adverse event – number (%) 32 (71.1) 21 (50.0) Common Grade 2 to 4 adverse event2 – number (%) Diarrhea 2 (4.4) 0 Nausea 2 (4.4) 0 Vomiting 3 (6.7) 2 (4.8) Gastritis 3 (6.7) 0 Jaundice 1 (2.2) 3 (7.1) Oral herpes 2 (4.4) 1 (2.4) Herpes zoster 0 2 (4.8) Varicella 2 (4.4) 0 Pneumonia 2 (4.4) 2 (4.8) Pneumocystis jiroveci pneumonia 2 (4.4) 0 Sinusitis 3 (6.7) 1 (2.4) Acute sinusitis 1 (2.2) 2 (4.8) Anorexia 2 (4.4) 0 3 Osteopenia 3 (6.7) 1 (2.4) Dizziness 3 (6.7) 2 (4.8) Adverse event related to study drug – number (%) Common adverse event related to study drug2 – number (%) Vomiting Osteopenia3 12 (26.7) 6 (14.3) 4 (8.9) 3 (6.7) 0 1 (2.4) Adverse event leading to permanent discontinuation of study drug – number (%) Vomiting 1 (2.2) 0 1 (2.2) 0 Serious adverse event – number (%) Common serious adverse event2 – number (%) Pneumonia Pneumocystis jiroveci pneumonia Sinusitis Serious adverse event related to study drug 10 (22.2) 3 (7.1) 3 (6.7) 2 (4.4) 2 (4.4) 0 1 (2.4) 0 0 0 Grade 3 or 4 laboratory abnormality – number (%) Absolute neutrophil count < 750 cells/mm3 Alanine aminotransferase > 5 x ULN Calcium < 7.0 mg/dL Creatine kinase > 10 x ULN Total bilirubin > 2.5 x ULN Fasting total cholesterol >300 mg/dL 1 7 (15.6) 0 1 (2.2) 0 4 (8.9) 0 2 (4.8) 1 (2.4) 0 1 (2.4) 4 (9.5) 1 (2.4) Adverse event terms are from the Medical Dictionary for Regulatory Activities (MedDRA), version 11.1. The laboratory-abnormality grade was assigned on the basis of the 2004 Division of AIDS criteria (http://rsc.techres.com/Document/safetyandpharmacovigilance/Table_for_Grading_Severity_of_Adult_ Pediatric_Adverse_Events.pdf). ULN denotes upper limit of the normal range. 2 Common adverse events are defined as occurring in more than 3% in either group. 3 Osteopenia events include only those spontaneously reported by investigators. No specific definition for osteopenia were used in the protocol or given to the investigators.





