Diffusion and Osmosis Lab Report Guidelines Title: Background
advertisement

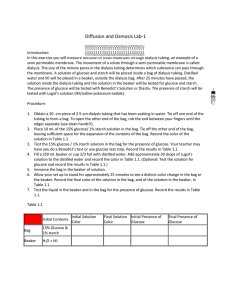
Diffusion and Osmosis Lab Report Guidelines Title: Background: Background about diffusion and osmosis. Purpose of the experiment. Hypothesis Part A: Predict what will move into and out of the bag (glucose, starch, water, IKI). Hypothesis Part B: Predict whether the bags will gain or lose mass when placed in distilled water. Data/Results: Data Table 1: Diffusion Initial Contents 15% glucose / 1% starch Bag H2O / IKI Beaker Solution Color Initial Final Clear Blue Brown Brown Data Table 2: Dialysis Bag Results – Lab Group Contents Initial Mass Final Mass 0.0 M 0.2 M 0.4 M 0.6 M 0.8 M 1.0 M + - Presence of Glucose Initial Final + + Mass Difference % Change in Mass 1.26 3.25 10.76 % Change in Mass = ((Final Mass – Initial Mass) / Initial Mass) x 100 Data Table 3: Dialysis Bag Results – Class Data Percent Change in Mass Group 1 Group 2 Group 3 Group 4 0.0 M 0.2 M 0.4 M 0.6 M 0.8 M 1.0 M Group 5 Group 6 Average 1.00 2.52 7.89 9.25 11.30 12.67 Graph1: Graph class data for percent change in mass. Include title and axis labels with units. Questions: PART A: 1. Which substances are entering the bag and which are leaving the bag? What experimental evidence supports your data? 2. Explain the results you obtained. Include the concentration differences and membrane pore size in your discussion. 3. How could this experiment have been modified so that quantitative data could be collected to show that water diffused into the dialysis bag? 4. Based on your observations, rank the following by size, beginning with the smallest: glucose (C 6H12O6), water, IKI, membrane pores, and starch. 5. What results would you expect if the experiment started with glucose and IKI inside the bag and only starch and water outside? PART B: 6. Explain the relationship between the change in mass and the molarity of sucrose within the dialysis bags. 7. Predict what would happen to the mass of each bag in this experiment if all of the bags were placed in a 0.4 M sucrose solution instead of distilled water. Explain your response. 8. Why did you calculate the percent change in mass rather than simply using the change in mass? 9. A dialysis bag is filled with distilled water and then placed in a sucrose solution. The bag’s initial mass is 20 g and its final mass is 18 g. Calculate the percent change in mass, showing all calculations. 10. The sucrose solution in the beaker would have been ______ to the distilled water in the bag. ( isotonic hypertonic hypotonic) Conclusion: Re-state the purpose of the lab. List and discuss all of the data from part A. Then list and discuss the class averages for the percent change in mass for part B. Next, discuss any errors that may have occurred in this lab. Finally, provide a suggestion for improvement or extension of the lab.