Vapor Pressure Curves
advertisement

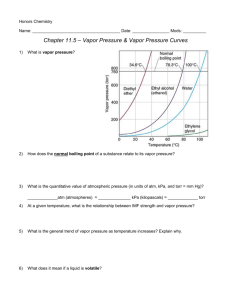
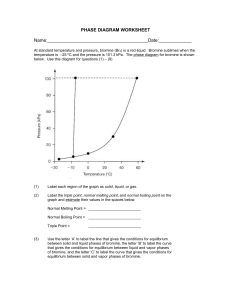
Honors Chemistry Name: ______________________________________ Date: ________________ Mods: __________ Chapter 11.5 – Vapor Pressure & Vapor Pressure Curves 1) What is vapor pressure? 2) How does the normal boiling point of a substance relate to its vapor pressure? 3) What is the quantitative value of atmospheric pressure (in units of atm, kPa, and torr = mm Hg)? _____________atm (atmospheres) = ______________ kPa (kilopascals) = _____________ torr 4) At a given temperature, what is the relationship between IMF strength and vapor pressure? 5) What is the general trend of vapor pressure as temperature increases? Explain why. 6) What does it mean if a liquid is volatile? Vapor Pressure Curves: Comparing Chloroform, Ethanol, Water, and Ethanoic Acid Directions: Use the vapor pressure curves below to answer the following questions: 1) What is the vapor pressure of chloroform at 50°C? _________________ 2) What is the boiling point of water when the external pressure is 30 kPa? _________________ 3) What is the normal boiling point of ethanol? _________________ 4) If ethanoic acid is boiling at 100°C, what is its external pressure? ___________________ 5) Comparing the curves, which substance (of the four given above) has the weakest IMFs? ___________________________________ 6) At an external pressure of 60 kPa, which substance would take the longest to heat to a boil? ___________________________________ 7) It has been proven that as elevation above sea level increases, the external atmospheric pressure will decrease. Since New Jersey is at sea level, water boils at the standard 100°C. If the elevation in Denver, CO is approximately 1 mile above sea level, will water boil at a higher or lower temperature than 100°C? ______________________________________ Chapter 11.6 – Phase Diagrams A phase diagram describes the temperature and pressure conditions at which a substance can exist as each state of matter. Phase Diagram of Bromine (Br2) [Note that the scales are distorted to emphasize some of the graph’s features] Directions: Answer the questions below using the phase diagram of bromine. At standard temperature and pressure, bromine is a red liquid. Bromine sublimes when its temperature is –25°C and its pressure is 101.3 kPa. 1) Label each region of the graph as solid, liquid, or gas. 2) Circle the words which accurately complete the sentence: Typically, solids exist at (high, low) pressures and (high, low) temperatures, while gases exist (high, low) pressures and (high, low) temperatures 3) Label the normal melting point, normal boiling point, and triple point on the graph and estimate their respective temperature values for Bromine in the space below: Normal melting point: Normal boiling point: __________________ __________________ Triple Point: _________________ 4) Use the letter “A” to label the line that gives the conditions of equilibrium between solid and liquid phases of bromine. This line represents the phase change known as ______________________. 5) Use letter “B” to label the line that gives the conditions of equilibrium between liquid and gas phases of bromine. This line represents the phase change known as ______________________. 6) Use the letter “C” to label the line that gives the conditions of equilibrium between solid and gas phases of bromine. This line represents the phase change known as ______________________. 7) Use arrows labeled “M”, “V” and “S” to label the portions of the graph where melting, vaporization, and sublimation occur, respectively 8) Describe how the melting point of bromine changes with the external pressure. 9) Based on the slope of the melting-point equilibrium line and the fact that higher pressure (at the same temperature) always favors the more dense state of matter, would you characterize the solid phase of bromine as more or less dense than the liquid phase of bromine? 10) What is the boiling point of bromine when the external pressure is 75 kPa? __________________ 11) Explain the significance of the triple point. 12) Complete the following sentences by circling the correct word in the parenthesis: a. Bromine gas at 15°C will (condense, sublime) when the pressure is raised from 10 to 50 kPa. b. Br2 liquid at 70 kPa (vaporizes, freezes) when the temperature is decreased from 20 to –15°C. c. Bromine gas at –20°C will undergo (sublimation, deposition) if the pressure is increased from 0 kPa to 10 kPa. Phase Diagrams: Comparing H2O (left) and CO2 (right) Directions: Answer the following questions regarding the two phase diagrams of H2O and CO2 above: 1) _____________________ State of matter water at 0.8 atm and 50ºC 2) _____________________ Phase change for CO2 as temperature increases from -90ºC to RT at 1atm. 3) _____________________ Which substance has the higher ΔH of vaporization? 4) _____________________ Phase change for CO2 as ΔT goes from -40ºC to 100ºC at 6atm. 5) _____________________ For CO2 which phase has the greater density, liquid or solid? 6) _____________________ At what minimum temperature and pressure can H2O exist as a liquid? 7) _____________________ Effect of increasing pressure on the state of matter of H 2O at 1 atm and 0ºC? 8) _____________________ Effect of increasing pressure on the boiling points of water and CO 2? 9) _____________________ What state of matter is CO2 at 20 atm and -70ºC? 10) _____________________ Temperature of the critical point of H2O (no liquid/gas phase boundary exists) 11) _____________________ Which of the two substances has the greater vapor pressure at 25ºC? 12) _____________________ Pressure must be (increased/decreased) in order to liquefy water at 140ºC. 13) _____________________ Which substance is more volatile? 14) _____________________ Process in which CO2 is cooled at 1atm from RT to -100ºC 15) _____________________ For H2O, which phase has a greater density, liquid or solid? 16) _____________________ Increased pressure will (lower/raise) the melting point of water. 17) _____________________ What IMFs account for water’s high boiling point? 18) _____________________ Does CO2 have a normal melting point? Explain in the space below. IMFs and Changes in States of Matter 1) The normal boiling point of a liquid is the temperature at which the vapor pressure of the escaping liquid molecules is equal to (or greater than) standard atmospheric pressure of 101.3 kPa. Liquids that have _____________________ intermolecular attractions must be heated to a higher temperature before boiling will occur. The boiling point of a liquid is lowered when the atmospheric pressure is _____________________, such as in the Rocky Mountains (high above sea level). When atmospheric pressure increases, such as in a pressure cooker, the temperature can get well above 100°C before water reaches its boiling point. Therefore, food cooks faster in a pressure cooker or covered pot because it is being cooked at a _______________________ temperature. Directions: Answer the following questions using the graph above: 2) What is the boiling point of water at an external pressure of 60 kPa? _______________________ 3) a. Which liquid has the strongest intermolecular forces? ______________________ b. What type of IMFs hold molecules of the substance together? ________________________________ 4) a. Which liquid has the weakest intermolecular forces? _______________________ b. What type of IMFs hold molecules of this substance together? ________________________________ 5) What is the vapor pressure of methanol, CH3OH, at a temperature of 25°C? _______________________ 6) Many chefs add salt to water when they are cooking. Beside the addition of flavor, what effect does the addition of salt, NaCl, have on the normal boiling point of water? Explain this in terms of IMFs exhibited between pure boiling water versus boiling water with salt. 7) A phase diagram summarizes the relationships among temperature, pressure, and the physical state of a particular substance. Each line represents the conditions at which a _________________ change occurs. There is a point where all three states of matter are in equilibrium with each other. This is called the ________________ point. Directions: Answer the following questions using the phase diagram for water shown above: 8) What happens to ice at 0°C when the blade from an Olympic ice skater increases the pressure exerted on the ice to 450 kPa? 9) Explain what changes occur when an ice cube at –5°C is placed in a vacuum and the atmospheric pressure is reduced to almost 0 kPa. 10) A particular substance has a triple point that occurs at 518 kPa and –56.4°C. Sketch a phase diagram of this substance based on this its triple point. What state of matter will this substance exist in at normal laboratory conditions (standard temperature and pressure)? 11) Thinking about phase diagrams, describe 2 changes you would make in laboratory experimental conditions in order to liquefy any gas.