Atoms, Molecules, and Ions
advertisement

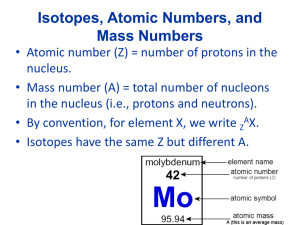
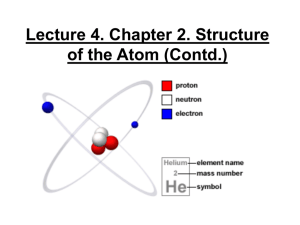
Chemistry 100 Chapter 2 Atoms, Molecules and Ions Law of Conservation of Matter By 1800, chemists had noted that the mass of reactants equals the mass of products - provided you capture any escaping gas Matter is not created or destroyed in a chemical reaction Law of Constant Composition Chemists (notably Proust) The relative amount (percentage) of each element in a compound was the same no matter how the compound was made These two laws lead Dalton to revive the Atomic Theory Matter is made up of small, indivisible particles Dalton’s Atomic Theory An element is composed of atoms. All atoms of a given element are the same. Atoms of different elements are different and have different properties. Dalton’s Theory-II Atoms are not changed, created or destroyed in a chemical reaction. Compounds are the combination of more than one element. A given compound has the same relative number and kind of atoms. Law of Multiple Proportions Accorrding to Dalton’s Theory Two elements (A and B) form two distinct compounds The amounts of B combining with a fixed amount of A would be a small whole number ratio. Water: 1 g hydrogen + 8 g oxygen Hydrogen peroxide: 1 g hydrogen +16 g oxygen Atomic Structure Roentgen discovered X-rays (1895) Becquerel discovered radioactivity (1896) J. J. Thompson discovered the electron (1897) Rutherford classified radioactivity emissions: alpha (), beta () and gamma () Alpha, beta, gamma Rutherford’s findings: Alpha are positive particles (+2) ; heavier than electrons Beta are high speed electrons; negatively charged particles (-1) Gamma are neutral rays Alpha particles are nucleus of He atom Thompson’s Model “Plum pudding” model A cloud of positive charge holding the negatively charge electrons in place Rutherford’s alpha experiment Scattering of alpha particles by gold foil Most particles were undeflected Some were deflected by large angles Rutherford’s Explanation Rutherford’s Model Centre - the nucleus - is small but positively charged Most of the atom is empty spaces Electrons rotate about the nucleus like the solar system Modern Model Additional experiments showed Nucleus consists of protons (positive) and neutrons (neutral) Electrons (negative) exist around the nucleus Number of protons = number of electrons Mass of Elementary Particles Protons and neutrons have a mass of about 1 amu Electrons have very small mass Most of the mass of an atoms comes from nucleus (1 amu is 1.66054 10-24 grams) Quantum Mechanical Model Atomic Mass & Atomic Number A is Mass Number, protons + neutrons A Sy Z Z is Atomic Number, number of protons Isotopes All atoms of a given element have the same number of protons. All Carbon atoms have 6 protons (and 6 electrons). The number of protons is different for each element. Atoms of a given element that differ in the number of neutrons are called isotopes. Examples : carbon-12 and carbon-14 12 6 C 14 6 C Atomic Masses By international agreement, the carbon-12 atom is defined as having a mass of exactly 12 atomic mass units (amu’s). All atomic masses are referenced to this standard. The Periodic Table A typical entry in the periodic table Atomic number 20 Ca 40.078 Atomic mass Periodic Table (II) Elements in the periodic table are arranged in Groups or families – they have similar chemical and physical properties Metals – towards the left Nonmetals – towards the right Metalloids – in the middle region Atomic Masses in the Periodic Table Question: why is the mass of C in the periodic table reported as 12.01 amu and not as 12.000 … amu, exactly? Another example: the atomic mass of Cl is = 35.453 amu’s. We would expect Cl to be 35 amu? Ionic Compounds Tables of common ions in textbook (pages 60 and 63). Ionic compounds Cation name followed by anion name, e.g., sodium bromide (NaBr) Multiple ion types FeCl2 – iron (II) chloride FeCl3 – iron (III) chloride Binary Molecular Compounds Binary compounds containing two nonmetals name of the first element in the formula followed by the stem of the name of the second element with the suffix -ide. The number of atoms of each element in the compound is indicated by a prefix. mono di tri tetra 1 2 3 4 Some common names must be committed to memory. Examples – nitrous oxide, N2O, and nitric oxide, NO. Formulas and Names of Acids An acid usually is a compound of hydrogen and a nonmetal or a polyatomic anion. Treat the hydrogen atoms of the acid as H+ ions. For acids containing monatomic anions, When these acids are found in water solution, add the prefix hydro- and the suffix -ic to the stem of the name of the anion Hydrofluoric acid (HF), hydrochloric acid (HCl) Acids From Polyatomic Anions If the anion name ends in ‘ate’, the ‘ate’ in the name of the anion is replaced by ‘ic acid ’ The acid of the sulfate ion is sulfuric acid (H2SO4) The acid of the nitrate ion is nitric acid (HNO3) If the anion names end in ‘ite’, we change the suffix to –ous and add the word acid. The acid of the nitrite is called nitrous acid, HNO2 The acid of the hypochlorite ion is called hypochlorous acid, HClO Average Atomic Masses Most elements in nature exist as mixtures of isotopes. Atomic masses reported in the periodic table - weighted averages of the different isotopes. The amount of each isotope in a sample of as an element may vary considerably with the source of the sample. This is the reason why some elements in the periodic table have few significant figures for their mass. Organic Compounds Many organic compounds have complex three dimensional structures chains, and/or rings branches. The highlighted groups are called functional groups. They are primarily responsible for the chemical and some physical properties of the molecules. Alkanes Methane (CH4) H C H Ethane (C2H6) Propane (C3H8) H H H H C C H H H H H C Butane (C4H10) H H C C H H H H H H H H H C H C C C H H H H H Alcohols Methanol (CH3OH) Ethanol (CH3CH2OH) 1-Propanol (CH3CH2CH2OH) 2-propanol (CH3CH(OH)CH3) H H H C OH H H H C C H H OH H H C C C OH H H H H H OH H C C H H C H H H H Organic Amines Methyl Amine (CH3NH2) H H Dimethyl amine ((CH3)2NH) Ethyl Amine (CH3CH2NH2) C NH2 H CH3 HN CH3 H H H C C H H NH2 Organic Acids Formic (Methanoic) acid (HCOOH) Acetic (Ethanoic) Acid (H3COOH) Propionic (Propanoic) Acid (H3CCH2COOH) O C OH H H O H C C H OH H O C C C OH H H H H