helpful notes for naming
advertisement

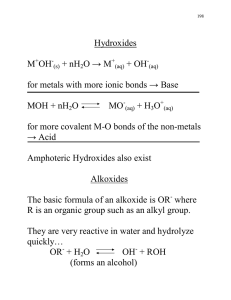
2.8 Naming Inorganic Compounds • Cations formed from a metal have the same name as the metal • Metal ions with more than 1 charge: Examples: Cu+ = copper (I) (old: cuprous) Pb+2 = lead (II) (old: plumbous) Cu+2 = copper (II) (old: cupric) Pb+4 = lead (IV) (old: plumbic) Fe+2 = iron (II) (old: ferrous) Sn+2 = tin (II) (old: stannous) Fe+3 = iron (III) (old: ferric) Sn+4 = tin (IV) (old: stannic) Mn+2 = manganese (II) (old: manganous) Mn+7 = manganese (VII) (old: manganic) • Cations formed from non-metals end in -ium Example: NH4+ ammonium ion. 11 • Monatomic anions (with only one atom) end in -ide Exceptions: Example: Cl- is chloride ion hydroxide (OH-), cyanide (CN-), peroxide (O22-) • Polyatomic anions (with many atoms) containing oxygen end in -ate or -ite (The one with more oxygen ends in -ate) Examples: NO3- is nitrate, NO2- is nitrite 21 • Polyatomic anions containing oxygen with more than two members in the series are named as follows (in order of decreasing oxygen): per-….-ate -ate -ite hypo-….-ite Text, P. 64 31 • Polyatomic anions containing oxygen with additional hydrogens: add “hydrogen” or “bi-” (one H) “dihydrogen” (two H), etc., to the name as follows: CO32- is the carbonate anion HCO3- is the hydrogen carbonate (or bicarbonate) anion PO4 -3 is the phosphate anion HPO4-2 is the hydrogen phosphate anion H2PO4- is the dihydrogen phosphate anion Note that the (-) charge decreases with each H that is added 41 Names and Formulas for Acids • Acids have the general form HX • The names of acids are related to the names of anions (X): -ide becomes hydro-….-ic acid -ate becomes -ic acid -ite becomes -ous acid 51 Text, P. 61 Names and Formulas of Binary Molecular Compounds • Binary molecular compounds are composed of 2 nonmetals • The most metallic element is usually written first (farthest left on the periodic table) • Exception: NH3 • If both elements are in the same group, the lower one is written first • Greek prefixes are used to indicate the number of atoms 71 Text, P. 62 Use a prefix to state how many of each atom are in the formula Don’t use “mono” for 1st element When adding a prefix, the “ao” combination is truncated “tetroxide” “heptoxide”