Learning Targets for the MOLE Unit
advertisement
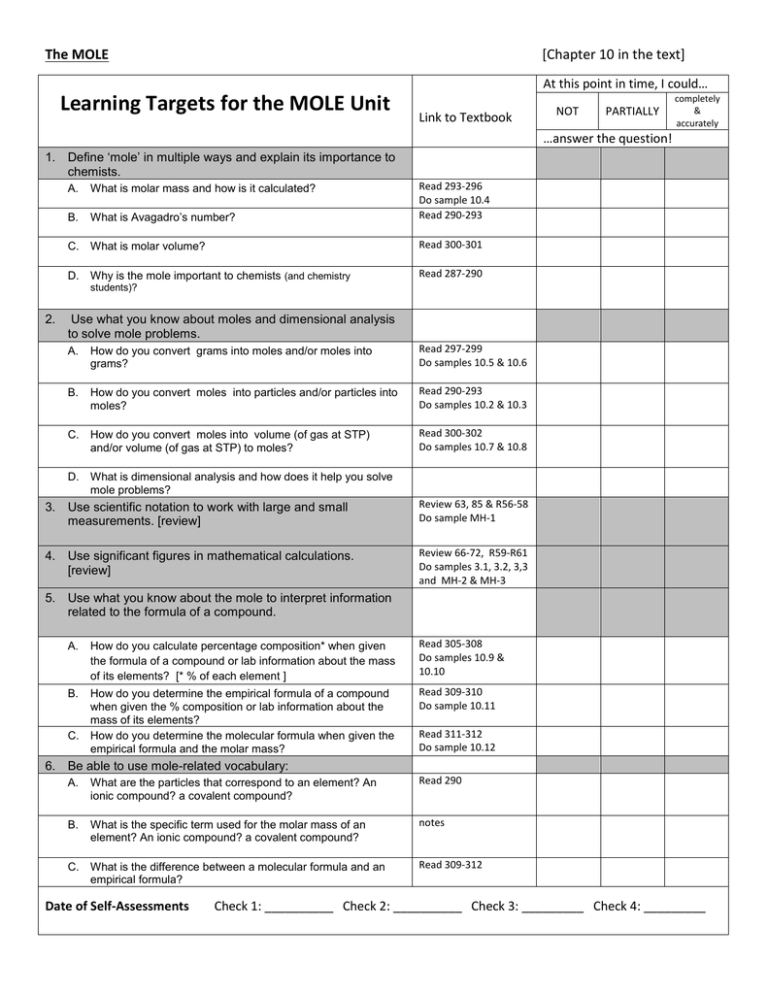
The MOLE [Chapter 10 in the text] At this point in time, I could… Learning Targets for the MOLE Unit Link to Textbook NOT PARTIALLY completely & accurately …answer the question! 1. Define ‘mole’ in multiple ways and explain its importance to chemists. A. What is molar mass and how is it calculated? B. What is Avagadro’s number? Read 293-296 Do sample 10.4 Read 290-293 C. What is molar volume? Read 300-301 D. Why is the mole important to chemists (and chemistry Read 287-290 students)? 2. Use what you know about moles and dimensional analysis to solve mole problems. A. How do you convert grams into moles and/or moles into grams? Read 297-299 Do samples 10.5 & 10.6 B. How do you convert moles into particles and/or particles into moles? Read 290-293 Do samples 10.2 & 10.3 C. How do you convert moles into volume (of gas at STP) and/or volume (of gas at STP) to moles? Read 300-302 Do samples 10.7 & 10.8 D. What is dimensional analysis and how does it help you solve mole problems? 3. Use scientific notation to work with large and small measurements. [review] Review 63, 85 & R56-58 Do sample MH-1 4. Use significant figures in mathematical calculations. [review] Review 66-72, R59-R61 Do samples 3.1, 3.2, 3,3 and MH-2 & MH-3 5. Use what you know about the mole to interpret information related to the formula of a compound. A. How do you calculate percentage composition* when given the formula of a compound or lab information about the mass of its elements? [* % of each element ] Read 305-308 Do samples 10.9 & 10.10 B. How do you determine the empirical formula of a compound when given the % composition or lab information about the mass of its elements? How do you determine the molecular formula when given the empirical formula and the molar mass? Read 309-310 Do sample 10.11 C. Read 311-312 Do sample 10.12 6. Be able to use mole-related vocabulary: A. What are the particles that correspond to an element? An ionic compound? a covalent compound? Read 290 B. What is the specific term used for the molar mass of an element? An ionic compound? a covalent compound? notes C. What is the difference between a molecular formula and an empirical formula? Read 309-312 Date of Self-Assessments Check 1: __________ Check 2: __________ Check 3: _________ Check 4: _________










