Written Assignment 3 Submit your solutions to the following
advertisement

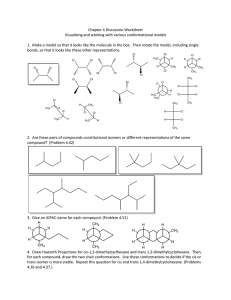
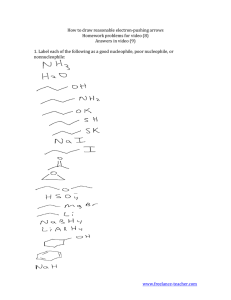
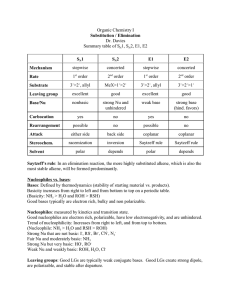
Written Assignment 3 Submit your solutions to the following problems. Show all work where applicable. Answers alone are not acceptable. 1. For the following compounds, write structural formulas and IUPAC names for all possible isomers having the identical number of multiple bonds: a. C5H8 (two double bonds) b. C6H10 (one triple bond) 2. Write a structural formula for each of the following compounds: a. b. c. d. 1,3-heptadiene 3-bromocyclohexene allyl bromide 2,3-dimethyl-1,3-cyclohexadiene 3. Which of the following compounds can exist as cis-trans isomers? If such isomerism is possible, draw the structures in a way that clearly illustrates the geometry. a. 2-butene b. 1-bromobutene c. 1-chloro-1,3-pentadiene 4. Write an equation for the reaction of 3-methyl-2-pentene with each of the following reagents: a. hydrogen bromide b. BH3 followed by H2O2, OH¯ c. hydrogen (Pt catalyst) d. KMnO4, OH¯ e. bromine f. H2O, H+ 5. What reagent will react by addition to what unsaturated hydrocarbon to form each of the following compounds? 6. Which of the following reagents is an electrophile? Which is a nucleophile? a. Br¯ b. BF3 7. Explain in your own words why the water molecule can act either as an electrophile or as a nucleophile. 8. The acid-catalyzed hydration of 1-ethylcyclopentene gives 1-ethylcyclopentanol. Write every step in the mechanism of this reaction. 9. Draw the resonance contributors to the carbocation Does the ion have a symmetric structure? 10. Adding one equivalent of sulfuric acid to 2,4-hexadiene gives two products. Give the structures, and write all the steps in a reaction mechanism that explains how each product is formed. 11. Predict the product of this Diels-Alder reaction. 12. Write an equation that clearly shows the structure of the alcohol obtained from the sequential hydroboration and H2O2/OH¯ oxidation of 1-methylcyclohexene. 13. Write equations to show how 2-methyl-1-pentene could be converted to a. 2-hydroxy-2-methylpentane b. 1-hydroxy-2-methylpentane 14. Write equations for the following reactions: a. 2-pentyne + H2 (1 mol, Lindlar's catalyst) b. 1-butyne + HBr (2 mol)