14.1 Introduction to Haloalkanes
advertisement

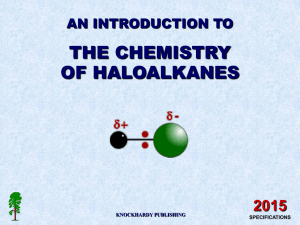
14.1 Introduction to Haloalkanes Learning Objectives: 1. Be able to name halogenalkanes. 2. Describe the physical properties of halogenalkanes. 3. Explain the trend in bond polarity. 4. Explain the trend in reactivity. Task Draw the skeletal formula and structural formula for the following: - 1-iodopentane 2-bromobutane 3-chloropentane 2-chloro-3-methylbutane 1-iodopentane I 2-bromobutane 3-chloropentane 2-chloro-3-methylbutane Cl tetrachloromethane iodomethane dibromodifluoromethane 1,1-diiodoethane 1-chloro-3-fluoro-2-iodopropane Primary, secondary and tertiary A chain of carbon atoms can be represented by R when drawing the structure. This is referred to as an R group. Primary (1°) halogenoalkanes have one R group attached to the carbon linked to the halogen. Secondary (2°) halogenoalkanes have two R groups attached to the carbon linked to the halogen. Tertiary (3°) halogenoalkanes have three R groups attached to the carbon linked to the halogen. Physical Properties – Boiling Point • Explain the trend in boiling points of haloalkanes: Why are fluorides not on the chart? Physical Properties – Boiling Pt. • Explain the trend in boiling points of haloalkanes: Physical Properties – Solubility • The carbon-halogen bond is polar (halogens more electronegative than carbon). • But not polar enough to be soluble in water. • Haloalkanes are… –soluble in organic solvents –insoluble in water Bond Polarity • Difference in electronegativity makes the C-X bond polar. Carbon Reactivity: Bond Polarity vs. Bond Enthalpy • Two factors determine the reactivity of haloalkanes – Bond polarity – Bond enthalpy Bond enthalpy is the more important factor, reactivity increases DOWN the group. 14.2 Nucleophilic Substitution Learning Objectives: 1. Define the term nucleophile and name some examples. 2. Describe the nucleophilic substitution reaction with haloalkanes. 3. Draw the mechanism for a nucleophilic substitution reaction. Carbon-Halogen Bond is Polar • The partial positive charge on the carbon atom (electron deficient) attracts species with a negative charge (electron rich). Nucleophiles • Nucleophiles are electron rich and are attracted to the partial positive charge on the carbon. Have lone pairs attached to an electronegative atom. • Nucleophiles = electron pair donors Examples: δOH¯ CN¯ NH3 δH 2O Nucleophilic Substitution Mechanism 1. Lone pair on nucleophile “attacks” the δ+ carbon and forms a dative covalent bond. 2. The halogen takes both electrons from the C-X bond and becomes the leaving group (X-). “curly arrow” represent movement of electron pairs NUCLEOPHILIC SUBSTITUTION AQUEOUS SODIUM HYDROXIDE ANIMATED MECHANISM Nucleophilic Substitution – OH• Haloalkanes react with hydroxides to produce alcohols and halide ions. Nucleophilic Substitution – CN• Haloalkanes react with cyanide ions to produce nitriles and halide ions. Nucleophilic Substitution – NH3 • Haloalkanes react with ammonia to produce amines and halide ions. 1) 2) 14.3 Elimination Reactions Learning Objectives: 1. Describe the elimination reaction of haloalkanes. 2. Draw the mechanism for an elimination reaction. 3. Describe the conditions that would favor elimination over substitution. Elimination Mechanism 1. OH- removes a H+ ion from the haloalkane to form water. 2. The electrons in the C-H bond now form a CC double bond. 3. The C now has 5 bonds so the halogen takes the electrons from the C-X bond and is eliminated as a halide ion. ELIMINATION ANIMATED MECHANISM Mixture of elimination products If the carbon chain is four or more carbons in length and the halogen is not attached to a terminal carbon, a mixture of positional isomers may be formed. attack at A but-2-ene A B attack at B but-1-ene Conditions: Substitution vs. Elimination Substitution • Cold hydroxide • Aqueous solution • Primary haloalkanes • OH- acts as a nucleophile Elimination • Hot hydroxide • Ethanolic solution (NO WATER) • Tertiary haloalkanes • OH- acts as a base (proton acceptor) 14.4 Formation of Haloalkanes Learning Objectives: 1. Define the term free radical. 2. Describe the reaction mechanism for freeradical substitutions. Free Radical • Free radical = species with an UNPAIRED electron . Cl Free Radical Substitution 1. Initiation (free radicals are formed) 2. Propagation (free radicals react to form additional free radicals) 3. Termination (free radicals react and are removed) Initiation • UV light provides the energy needed to break the Cl-Cl bond. Propagation • Free radicals are EXTREMELY reactive. They react with molecules to form new free radicals. This is a chain reaction. Termination • When two free radicals react it removes the free radicals and terminates the chain reaction. • There are multiple termination products. Example: CFC’s and the Ozone Layer • Chlorofluorocarbons (CFC’s) are haloalkanes. • They are known to cause damage to the ozone layer (which protects us from harmful UV radiation). • Ozone is destroyed through a free radical substitution reaction. Read pg. 193 and 195 and make notes on CFCs and the ozone layer including the chemical equations for the free radical reactions.