5.1 The Nature of Light
advertisement

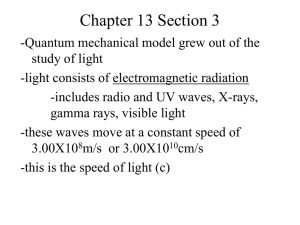
5.1 THE NATURE OF LIGHT Chemistry Ms. Pollock 2013 - 2014 INTRODUCTION • • Understanding of Rutherford’s model requires understanding of light 1600s debate about how light travels Isaac Newton – light beam of particles ● Christian Huygens – light wave of energy ● • Neither hypothesis dominant until 1864 wave model of light (James Clerk Maxwell) accepted by many ● Relationship between magnetism and electricity ● Debate renewed by Max Planck sixty years later INTRODUCTION Above left: Newton; above right: Huygens; below left: Planck; below right: Maxwell THE WAVE FORM OF ENERGY • • • Wave model of electromagnetic radiation similar to waves on rope Moving rope up and down with one end secured, motion passed from one part of rope to next Rope gains wave shape THE WAVE FORM OF ENERGY • • • • Crest – highest point of wave Trough – lowest point of wave No horizontal motion of particles Wavelength – distance from one crest to the next crest; symbol λ THE WAVE FORM OF ENERGY • • • • Amplitude – maximum height of wave Velocity – distance traveled by wave in one second (unit m/s, symbol ν) Frequency – number of cycles that pass a given point per unit of time (unit 1/s or s-1) Hertz – wave cycles per second (symbol ƒ) THE WAVE FORM OF ENERGY • • • • • Velocity, wavelength and frequency related ν = ƒλ Velocity = frequency X wavelength What is the wavelength of a water wave if its velocity is 5.0 m/s and its frequency is 2.0 s-1? λ = ν = 5.0 m/s = 2.5 meters ƒ 2.0 s-1 ELECTROMAGNETIC WAVES • • • • • Form of electromagnetic radiation with electric and magnetic fields moving at the speed of light Carry energy from one place to another and are like waves on rope Do not require medium Energy traveling in straight line along path of wave Associated oscillating electrical field and oscillating magnetic field ELECTROMAGNETIC WAVES ELECTROMAGNETIC WAVES • • • • • Light waves still characterized by wavelength, frequency, and velocity Velocity of all electromagnetic waves in vacuum same value (3.00 X 108 m/s), symbolized by c c = ƒλ What is the wavelength of an electromagnetic wave traveling in air whose frequency is 1.00 X 10 14 s-1? λ = c = 3.00 X 108 m/s = 3.00 X 10-6 m ƒ 1.00 X 1014 s-1 THE ELECTROMAGNETIC SPECTRUM • • • • • Frequency related to energy and amplitude of electromagnetic waves Frequency able to be converted to energy by multiplying by Planck’s constant (h = 6.6 X 10-34J⋅s) E = hƒ Wide range of frequencies, wavelengths, and energies in spectrum Electromagnetic spectrum – range of all possibilities of electromagnetic radiation THE ELECTROMAGNETIC SPECTRUM