Lab Activity
advertisement

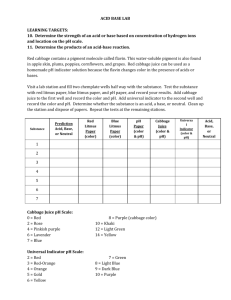
Lab Activity Acids and Bases Scientific Title: The effect of various solutions on pH level. Problem Statement: How can a pH indicator determine the pH level of substances? Hypothesis: If an indicator is used, then the acidity and alkalinity(base) can be determined, because various colors will be observed indicating the pH level of the substance due to the number of hydrogen ions (H+). Variables: Refer to the title, problem statement, and hypothesis to identify the variables. Independent variable Dependent variable Constant/Control- Materials: Cabbage juice 13 test tubes 1Test tube rack Variety of solutions (list all substances) Vinegar, baking soda, gingerale, antacid, orange juice, tap water, pure water, salt water, windex, tea, ammonia, milk, liquid soap 13 Pipettes Litmus paper (blue and red) Procedures: 1. Place the solutions in the test tubes. 2. Add 3-4 drops of cabbage juice to each test tube. 3. Observe and record observations on the data table. 4. Use the litmus paper (red and blue) to determine whether the substances are a base, an acid or neutral based on the color of the paper. 5.Record your observations. Data Table: Copy the data table for a total of 13 solutions/substances. Complete column 1 by listing all of the 13 solutions in order as listed under materials. Complete the second column by hypothesizing whether or not the solution/substance is an acid, a base or is it neutral. Column #3, write the color observed and the pH level (the number) Take a picture of the test tubes with the substances before and after the indicator is added. Number the test tubes 1-6 and 7-12. Do not include #13, which was the dish liquid in the cup. Include the pictures after the 7 data questions and before the conclusion paragraphs. #10 Tea #13 Dish liquid Data Analysis: Please write the 7 analysis questions and leave space in between each for the answer(s). Answer to #6 (write the first bullet and choose one of the other 3 bullets to include as part of the answer to #6.) How does this relate to pH? The numbers on the pH scale run from 0 to 14 Substances with lower pH's have much much more hydrogen, or H+, than substances with higher pH's. For example: Vinegar, with a pH of 3, has ten times more H+ than lemon juice, with a pH of 4. Lemon juice, with a pH of 4, has ten times more H+ than aspirin water, with a pH of 5. Aspirin water, with a pH of 5, has ten times more H+ than milk, with a pH of 6. Conclusion: Paragraph 1 -10 points The purpose of this lab was to determine how a pH indicator is used to determine the pH level of various substances. The pH indicator determines the acidity and alkalinity (base) of a solution. If the pH level is less than seven,the substance is an acid. If the pH is greater than seven, the substance is a base. If the substance pH level is seven, it is considered neutral. The procedures used was to first add the 13 substances to 13 test tubes. The second step was the addition of the cabbage juice, which was the indicator. The observations were then recorded on the data table. Both the color observed and the pH level was recorded. The independent variable is this activity was the substances. The dependent variable was the pH level. The constant was the cabbage juice. Conclusion : Paragraph 2 – 20 points In this paragraph include your answers from the data analysis questions. Include 1, 4 and 5; also what substances where acidic and which were basic. Explain how you were able to make that determination. Include the other 3 indicators that are used to identify an acid or a base from your “Properties of Acids and Bases” notes. Include the colors that are observed from these indicators that will identify an acid from a base. Conclusion: Paragraph 3- 15 points Include whether or not your hypothesis was supported and explain. Also, what real-life or real-world applications are related to the concepts of acids and bases.You can refer to your 3 sets of notes for the answers, especially the notes on “Writing and Naming Acids and Bases” from teacher web. Include 4 applications. Two for acids and two for bases.