Chapter 1-
advertisement

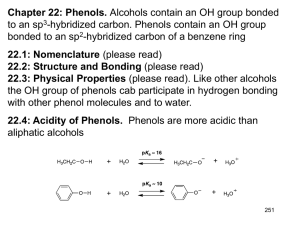
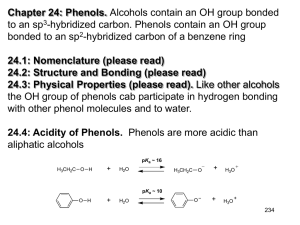
Chapter 21 Phenols and Aryl Halides: Nucleophilic Aromatic Substitution Structure and Nomenclature of Phenols Phenols have hydroxyl groups bonded directly to a benzene ring Naphthols and phenanthrols have a hydroxyl group bonded to a polycyclic benzenoid ring Chapter 21 2 Nomenclature of Phenols Phenol is the parent name for the family of hydroxybenzenes Methylphenols are called cresols Chapter 21 3 Synthesis of Phenols Laboratory Synthesis Phenols can be made by hydrolysis of arenediazonium salts Chapter 21 4 Industrial Syntheses 1. Hydrolysis of Chlorobenzene (Dow Process) Chlorobenzene is heated with sodium hydroxide under high pressure The reaction probably proceeds through a benzyne intermediate (Section 21.11B) 2. Alkali Fusion of Sodium Benzenesulfonate Sodium benzenesulfonate is melted with sodium hydroxide Chapter 21 5 3. From Cumene Hydroperoxide Benzene and propene are the starting materials for a three-step sequence that produces phenol and acetone Most industrially synthesized phenol is made by this method The first reaction is a Friedel-Crafts alkylation Chapter 21 6 The second reaction is a radical chain reaction with a 3o benzylic radical as the chain carrier Chapter 21 7 The third reaction is a hydrolytic rearrangement (similar to a carbocation rearrangement) that produces acetone and phenol A phenyl group migrates to a cationic oxygen group Chapter 21 8 Reactions of Phenols as Acids Strength of Phenols as Acids Phenols are much stronger acids than alcohols Chapter 21 9 Phenol is much more acidic than cyclohexanol Experimental results show that the oxygen of a phenol is more positive and this makes the attached hydrogen more acidic The oxygen of phenol is more positive because it is attached to an electronegative sp2 carbon of the benzene ring Resonance contributors to the phenol molecule also make the oxygen more positive Chapter 21 10 Distinguishing and Separating Phenols from Alcohols and Carboxylic Acids Phenols are soluble in aqueous sodium hydroxide because of their relatively high acidity Most alcohols are not soluble in aqueous sodium hydroxide A water-insoluble alcohol can be separated from a phenol by extracting the phenol into aqueous sodium hydroxide Phenols are not acidic enough to be soluble in aqueous sodium bicarbonate (NaHCO3) Carboxylic acids are soluble in aqueous sodium bicarbonate Carboxylic acids can be separated from phenols by extracting the carboxylic acid into aqueous sodium bicarbonate Chapter 21 11 Other Reactions of the O-H Group of Phenols Phenols can be acylated with acid chlorides and anhydrides Chapter 21 12 Phenols in the Williamson Ether Synthesis Phenoxides (phenol anions) react with primary alkyl halides to form ethers by an SN2 mechanism Chapter 21 13 Cleavage of Alkyl Aryl Ethers Reaction of alkyl aryl ethers with HI or HBr leads to an alkyl halide and a phenol Recall that when a dialkyl ether is reacted, two alkyl halides are produced Chapter 21 14 Reaction of the Benzene Ring of Phenols Bromination The hydroxyl group is a powerful ortho, meta director and usually the tribromide is obtained Monobromination can be achieved in the presence of carbon disulfide at low temperature Nitration Nitration produces o- and p-nitrophenol Low yields occur because of competing oxidation of the ring Chapter 21 15 Sulfonation Sulfonation gives mainly the the ortho (kinetic) product at low temperature and the para (thermodynamic) product at high temperature Chapter 21 16 The Kolbe Reaction Carbon dioxide is the electrophile for an electrophilic aromatic substitution with phenoxide anion The phenoxide anion reacts as an enolate The initial keto intermediate undergoes tautomerization to the phenol product Kolbe reaction of sodium phenoxide results in salicyclic acid, a synthetic precursor to acetylsalicylic acid (aspirin) Chapter 21 17 The Claisen Rearrangement Allyl phenyl ethers undergo a rearrangement upon heating that yields an allyl phenol The process is intramolecular; the allyl group migrates to the aromatic ring as the ether functional group becomes a ketone The unstable keto intermediate undergoes keto-enol tautomerization to give the phenol group The reaction is concerted, i.e., bond making and bonding breaking occur at the same time Chapter 21 18 Allyl vinyl ethers also undergo Claisen rearrangement when heated The product is a g-unsaturated carbonyl compound The Cope rearrangement is a similar reaction Both the Claisen and Cope rearrangements involve reactants that have two double bonds separated by three single bonds Chapter 21 19 The transition state for the Claisen and Cope rearrangements involves a cycle of six orbitals and six electrons, suggesting aromatic character This type of reaction is called pericyclic The Diels-Alder reaction is another example of a pericyclic reaction Chapter 21 20 Quinones Hydroquinone is oxidized to p-benzoquinone by mild oxidizing agents Formally this results in removal of a pair of electrons and two protons from hydroquinone This reaction is reversible Every living cell has ubiquinones (Coenzymes Q) in the inner mitochondrial membrane These compounds serve to transport electrons between substrates in enzymecatalyzed oxidation-reduction reactions Chapter 21 21 Aryl Halides and Nucleophilic Aromatic Substitution Simple aryl and vinyl halides do not undergo nucleophilic substitution Back-side attack required for SN2 reaction is blocked in aryl halides Chapter 21 22 SN2 reaction also doesn’t occur in aryl (and vinyl halides) because the carbon-halide bond is shorter and stronger than in alkyl halides Bonds to sp2-hybridized carbons are shorter, and therefore stronger, than to sp3-hybridized carbons Resonance gives the carbon-halogen bond some double bond character Chapter 21 23 Nucleophilic Aromatic Substitution by Addition- Elimination: The SNAr Mechanism Nucleophilic substitution can occur on benzene rings when strong electron-withdrawing groups are ortho or para to the halogen atom The more electron-withdrawing groups on the ring, the lower the temperature required for the reaction to proceed Chapter 21 24 The reaction occurs through an addition-elimination mechanism A Meisenheimer complex, which is a delocalized carbanion, is an intermediate The mechanism is called nucleophilic aromatic substitution (SNAr) The carbanion is stabilized by electron-withdrawing groups in the ortho and para positions Chapter 21 25 Nucleophilic Aromatic Substitution through an Elimination-Addition Mechanism: Benzyne Under forcing conditions, chlorobenzene can undergo an apparent nucleophilic substitution with hydroxide Bromobenzene can react with the powerful base amide Chapter 21 26 The reaction proceeds by an elimination-addition mechanism through the intermediacy of a benzyne (benzene containing a triple bond) Chapter 21 27 A calculated electrostatic potential map of benzyne shows added electron density at the site of the benzyne p bond The additional p bond of benzyne is in the same plane as the ring When chlorobenzene labeled at the carbon bearing chlorine reacts with potassium amide, the label is divided equally between the C-1 and C-2 positions of the product This is strong evidence for an elimination-addition mechanism and against a straightforward SN2 mechanism Chapter 21 28 Benzyne can be generated from anthranilic acid by diazotization The resulting compound spontaneously loses CO2 and N2 to yield benzyne The benzyne can then be trapped in situ using a Diels-Alder reaction Phenylation Acetoacetic esters and malonic esters can be phenylated by benzyne generated in situ from bromobenzene Chapter 21 29 Spectroscopic Analysis of Phenols and Aryl Halides Infrared Spectra Phenols show O-H stretching in the 3400-3600 cm-1 region 1H NMR The position of the hydroxyl proton of phenols depends on concentration In phenol itself the O-H proton is at d 2.55 for pure phenol and at d 5.63 for a 1% solution Phenol protons disappear from the spectrum when D2O is added The aromatic protons of phenols and aryl halides occur in the d 79 region 13C NMR The carbon atoms of phenols and aryl halides appear in the region d 135-170 Chapter 21 30