Properties of Water Stations Lab
advertisement

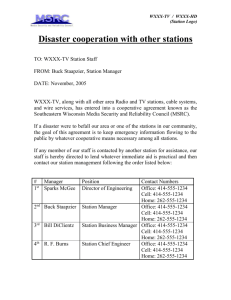
Name Class Date Properties of Water Stations Lab As a group, you will complete the laboratory experiment at each of the lab stations and record your observations and results for each station. Write your responses in complete sentences whenever possible. Follow the directions for each lab that are posted at each of the lab stations. Be sure to pay special attention to the purpose of each lab before beginning the lab. Station 1: Density 1. Write your observations of the ice cube in the water. 2. Summarize your thoughts about the impact on the world if ice were denser than water. 3. Water molecule drawing: Questions 4. Why does ice float rather than sink? 5. How would life in a lake be affected if ice sank and lakes froze from the bottom up? 3/22/16 8:00 AM 1 of 9 Oelfke (elf-ka) Station 2: Surface Tension Counting Drops on a Penny 6. Fill in the table below with the number of drops you added to the penny of each substance before the liquid spilled over. Substance Water Oil Soapy Water Number of Drops Comparing the Shape of a Drop 7. After placing a few drops of each of the liquids on the wax paper, draw what the drops look like from the side view. Be sure to capture the relative height/flatness of the drop. Water Oil Soapy Water Upside Down Water Trick 8. Write your observations of what occurred when the Erlenmeyer flask was turned upside down. Questions 9. What does a high surface tension do to the number of liquid molecules that can stay together? 10. Based on your evidence, compare the surface tension of water, oil, and soapy water. 11. Give a reason for the varying shapes of the water, oil, and soapy water. 12. Explain the reason for your observations of the upside down water in the Erlenmeyer flask. 3/22/16 8:00 AM 2 of 9 Oelfke (elf-ka) Name Class Date Properties of Water Stations Lab As a group, you will complete the laboratory experiment at each of the lab stations and record your observations and results for each station. Write your responses in complete sentences whenever possible. Follow the directions for each lab that are posted at each of the lab stations. Be sure to pay special attention to the purpose of each lab before beginning the lab. Station 3: Evaporation Rate and Specific Heat Evaporation Rate 13. Record the time for the water and isopropyl alcohol to evaporate. Substance Evaporation Time (seconds) Water Isopropyl alcohol 14. Does water or isopropyl alcohol have a higher specific heat? How do you know? 15. Which becomes hot first, the water or the pot? Explain why. Questions 16. Which substance, water or isopropyl alcohol, had the higher heat of vaporization? 17. Based on your results explain why water is a much more effective coolant than alcohol for the body. 18. Explain why this property of water is important to living organisms? 19. When going to the pool or beach, why does the water temperature feel cooler than the air temperature? 3/22/16 8:00 AM 3 of 9 Oelfke (elf-ka) 3/22/16 8:00 AM 4 of 9 Oelfke (elf-ka) Station 4: Adhesion/Cohesion 20. Fill in the diameters of the filter papers on the data table below. 21. Record the height of the liquid on the filter paper of different widths as you take your measurements. Height of Dye on Filter Paper Over Time Filter Paper Width (mm) 1 minutes 2 minutes 3 minutes 4 minutes 5 minutes Questions 22. Define adhesion in your own words. 23. Define cohesion in your own words. 24. Based on your evidence, what statement can you make about water’s speed of climbing filter paper of varying widths? 25. What does this mean about how fast water is able to “climb” tubes within plants? 3/22/16 8:00 AM 5 of 9 Oelfke (elf-ka) Name Class Date Properties of Water Stations Lab As a group, you will complete the laboratory experiment at each of the lab stations and record your observations and results for each station. Write your responses in complete sentences whenever possible. Follow the directions for each lab that are posted at each of the lab stations. Be sure to pay special attention to the purpose of each lab before beginning the lab. Station 5: Ability to Dissolve 26. Record your observations about how quickly and thoroughly each of the solutes dissolves in water and oil in the table below. Solutes Solvent Salt Sugar Iodine crystals Water Oil Questions 27. Summarize what you found in your experiment, based on your recorded observations. 28. Why do you think that some substances dissolve easier in one type of liquid than in another? 3/22/16 8:00 AM 6 of 9 Oelfke (elf-ka) Station 6: Liquid at Room Temperature Liquid at Room Temperature Data Activity Questions 29. What trends do you notice in the data table? Explain. 30. What is unusual about the most common pattern? Explain. 31. How does water compare to other substances? 3/22/16 8:00 AM 7 of 9 Oelfke (elf-ka) Name Class Date Properties of Water Stations Lab As a group, you will complete the laboratory experiment at each of the lab stations and record your observations and results for each station. Write your responses in complete sentences whenever possible. Follow the directions for each lab that are posted at each of the lab stations. Be sure to pay special attention to the purpose of each lab before beginning the lab. Analysis 32. List three things that you discovered about water. 33. List two ways that the characteristics of water help the body. 34. List two ways that characteristics of water help plants. 35. Which property of water is represented by each question? Explain your answer. A. How does water rise from the roots of a tree to the very top? B. How do insects walk on the water? C. Why does pool water feel so nice and cool on a hot summer day? D. Why does ice float rather than sink? E. Why is sweating a good way for our bodies to cool down? F. What happens to sugar when mixed in with hot or cold tea? 3/22/16 8:00 AM 8 of 9 Oelfke (elf-ka) 3/22/16 8:00 AM 9 of 9 Oelfke (elf-ka)