CRF / Source Documents
advertisement

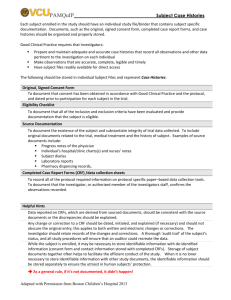
The Difference between Source Documents and CRF’s The primary purpose of the source documents is for the care of the study subject from a clinical perspective: Source documents are comprised of the following types of documents: Patient files -medical notes, summaries of physical findings, details of medical history, concurrent medications/devices and diseases are noted Recordings from automated instruments, traces-ECG, EEG, automatic BP readings X-ray films- actual film, faxed result or paper the result was written on when called in Laboratory Results- if called in the paper it is written on or fax of results Computer Databases - psychological tests requiring direct entry by patient on to computers or direct entry of patient information on to computers by physicians - If source data are entered directly on the computer, there must be a safeguard to ensure validation, including a signed and dated printout to use as backup records In fact, at each visit, the monitor (if applicable) should obtain a printout, which is signed and dated by the investigator, and which serves as the source document and is maintained at the study site. This process is necessary because most clinical settings do not have systems which are sophisticated enough to provide a secure and accurate data audit train, that is, a trail when and how data are changed, if at all, and have not been reliably validated (i.e. have SOPs and procedures to ensure the survival of the electronic data). The primary purpose of the CRFs is to collect research data: CRF’s are documents designated by the researchers to collect research data. It is important that CRFs only collect data necessary for the research question Collecting too little or too much data may be unethical or unscientific - thus the CRF is a biased document in the sense that only data which are relevant to the proposed hypothesis will be collected. CRFs (and other data collection forms) generally cannot substitute as source documents Data entered in CRFs should be supported by data in a source document. There are exceptions to this rule: o CRF may be used as source data when a reading is recorded exclusively for research and is not found elsewhere. (e.g. voluminous blood pressure readings, numerous psychiatric rating scales, etc.) o You may have a patient fill out a study questionnaire directly on the CRF; there would not be a separate source for this. This is only acceptable if the data would not normally be entered in the medical record, and if knowledge of such data is not required by the investigator or other clinicians who concurrently or subsequently treat the study subject.