Molecular geometry, polarity MULTIPLE CHOICE QUESTIONS
advertisement
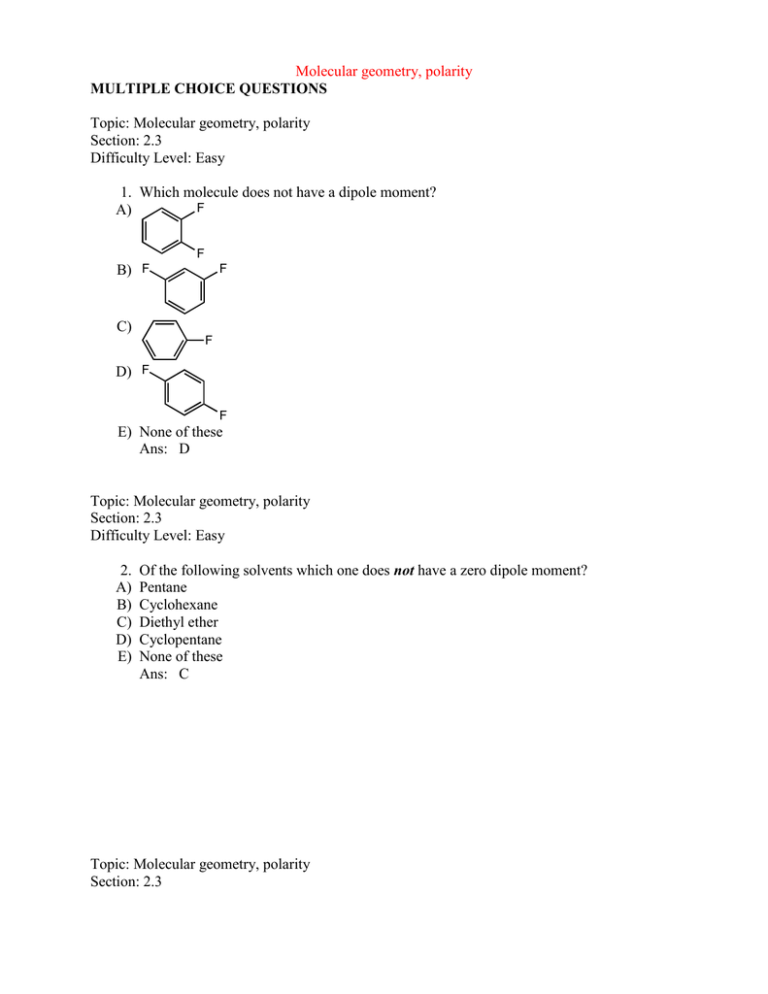
Molecular geometry, polarity MULTIPLE CHOICE QUESTIONS Topic: Molecular geometry, polarity Section: 2.3 Difficulty Level: Easy 1. Which molecule does not have a dipole moment? F A) F F B) F C) F D) F F E) None of these Ans: D Topic: Molecular geometry, polarity Section: 2.3 Difficulty Level: Easy 2. A) B) C) D) E) Of the following solvents which one does not have a zero dipole moment? Pentane Cyclohexane Diethyl ether Cyclopentane None of these Ans: C Topic: Molecular geometry, polarity Section: 2.3 Difficulty Level: Easy 3. A) B) C) D) E) Which molecule has a zero dipole moment? CH3Cl CH2Cl2 CHCl3 CCl4 None of these Ans: D Topic: Molecular geometry, dipole moment Section: 2.3 Difficulty Level: Easy 4. Which molecule would you expect to have no dipole moment (i.e., = 0 D)? A) CHF3 H B) F H C) :NF3 D) F H E) CH2F2 Ans: B F F H Topic: Molecular geometry, dipole moment Section: 2.3 Difficulty Level: Easy 5. Which molecule has a dipole moment greater than zero? F A) F H B) F C) H H F F H H H F D) More than one of these E) None of these Ans: D Topic: Molecular geometry, dipole moment Section: 2.3 Difficulty Level: Medium 6. Which of the following would have no net dipole moment ( = 0 D)? A) CBr4 B) cis-1,2-Dibromoethene C) trans:-1,2-Dibromoethene D) 1,1-Dibromoethene E) More than one of these Ans: E Topic: Molecular geometry, polarity Section: 2.3 Difficulty Level: Medium 7. For a molecule to possess a dipole moment, which following condition is necessary but not sufficient? A) Three or more atoms in the molecule B) Presence of one or more polar bonds C) A non-linear structure D) Presence of oxygen or fluorine E) Absence of a carbon-carbon double or triple bond Ans: B Topic: Molecular geometry, polarity Section: 1.5 and 2.3 Difficulty Level: Easy 8. A) B) C) D) E) Which molecule has a zero dipole moment? SO2 CO2 CO CHCl3 None of these Ans: B Topic: Molecular geometry, polarity Section: 1.5 and 2.3 Difficulty Level: Easy 9. A) B) C) D) Which molecule has a zero dipole moment? CO2 CH4 CH3CH3 E) All of these Ans: E Topic: Molecular geometry, polarity Section: 1.5 and 2.3 Difficulty Level: Medium 10. A) B) C) D) E) Which molecule has a dipole moment of zero? CHCl3 CH2Cl2 ClHC=CH2 trans-ClHC=CHCl None of these Ans: D Topic: Molecular geometry, polarity Section: 1.5 and 2.3 Difficulty Level: Medium 11. A) B) C) D) E) Which molecule would have a dipole moment greater than zero? BeCl2 BCl3 CO2 H2O CCl4 Ans: D Topic: Molecular geometry, polarity Section: 1.5, 1.6 and 2.3 Difficulty Level: Medium 12. A) B) C) D) E) A non-zero dipole moment is exhibited by: SO2 CO2 CCl4 BF3 Cl Cl Cl Cl Ans: A Topic: Molecular geometry, polarity Section: 1.5, 1.6, and 2.3 Difficulty Level: Hard 13. Of the following common organic solvents which one is predicted to have a smaller dipole moment? A) Chloroform, CHCl3 B) Acetone, (CH3)2CO C) Dimethylsulfoxide, (CH3)2SO D) Acetonitrile, CH3CN E) Methanol, CH3OH Ans: A Topic: Molecular geometry, Polarity Section: 2.3A Difficulty Level: Easy 14. Which molecule(s) has/have dipole moment(s) equal to zero? Cl A) B) Cl Cl C) Cl Cl Cl D) Cl Cl E) None of these have dipole moment equal to zero. Ans: C Topic: Functional groups Section: 2.4 Difficulty Level: Easy 15. What alkyl groups make up the following ether? O A) B) C) D) E) ethyl and phenyl propyl and benzyl ethyl and benzyl propyl and phenyl None of these Ans: C Topic: Functional groups Section: 2.4 Difficulty Level: Medium 16. What alkyl groups make up the following ketone? Ph O A) B) C) D) E) Phenyl, pentyl Hexyl, phenyl Benzyl, hexyl Benzyl, heptyl None of these Ans: C Topic: Functional groups Section: 2.4A Difficulty Level: Easy 17. What alkyl groups make up the following ether? O A) B) C) D) E) Isobutyl and methyl Methyl and butyl Ethyl and isopropyl Methyl and sec-butyl None of these Ans: D Topic: Functional groups Section: 2.4A Difficulty Level: Easy 18. What alkyl groups make up the following ether? O A) B) C) D) E) isobutyl and propyl propyl and butyl ethyl and isopropyl propyl and sec-butyl None of these Ans: A Topic: Functional groups Section: 2.4A Difficulty Level: Easy 19. What alkyl group is attached to the oxygen in the following ester? O O A) B) C) D) E) ethyl propyl sec-propyl isopropyl None of these Ans: D Topic: Functional groups Section: 2.4A Difficulty Level: Easy 20. What alkyl groups make up the following 3o amine? N A) B) C) D) E) sec-butyl, ethyl, propyl isobutyl, isopropyl, ethyl sec-butyl, ethyl, isopropyl butyl, ethyl, propyl None of these Ans: A Topic: Functional groups Section: 2.4A Difficulty Level: Easy 21. What alkyl groups are attached to the benzene ring in the following example? H3CH2CH2C A) B) C) D) E) ethyl, butyl ethyl, isobutyl proplyl, sec-butyl propyl, butyl None of these Ans: E Topic: Functional groups Section: 2.4B Difficulty Level: Medium 22. What common group is attached to both the ether and 3o amine in the following molecule? OCH2C6H5 NBn A) B) C) D) E) benzyl phenyl heptyl ethyl None of these Ans: A Topic: Functional groups Section: 2.4B Difficulty Level: Medium 23. What group makes up the following aldehyde (benzaldehyde)? C6H5CHO A) benzyl B) phenyl C) heptyl D) ethyl E) None of these Ans: B Topic: Functional groups Section: 2.5 Difficulty Level: Easy 24. What functional group is present in the following compound? Br A) B) C) D) E) o 1 alkyl bromide 2o alcohol 2o alkyl bromide 1o amine 3o alkyl bromide Ans: C Topic: Functional Groups Section: 2.5 Difficulty Level: Easy 25. Which is a 3 alkyl halide? F Br Cl I II III I Br IV A) B) C) D) E) I II III IV V Ans: B Topic: Functional groups Section: 2.5 Difficulty Level: Medium V 26. Which compound(s) contain(s) tertiary carbon atom(s)? F I II III Br OH OH IV A) B) C) D) E) V I, II, III I II, III I, IV V Ans: D Topic: Functional groups Section: 2.5 Difficulty Level: Medium 27. Which of these compounds is a secondary alkyl chloride? A) CH3CH2CH2CH2CH2Cl CH3 B) CH3CCH2CH3 Cl C) CH3CHCH2CH2CH3 Cl D) (CH3)2CHCHClCH3 E) Two of these Ans: E Topic: Functional groups, Isomerism Section: 1.3A and 2.5 Difficulty Level: Medium 28. A) B) C) D) E) How many 2º alkyl bromides, neglecting stereoisomers, exist with the formula C6H13Br? 4 5 6 7 8 Ans: C Topic: Isomers Section: 1.3A and 2.5 Difficulty Level: Medium 29. The number of unique open-chain structures corresponding to the molecular formula C3H5Cl is: A) 2 B) 3 C) 4 D) 5 E) 6 Ans: C Topic: Functional groups Section: 2.6 Difficulty Level: Easy 30. Which compound listed below is a secondary alcohol? A) CH3CHCH2CH3 OH CH CHCH B) 3 2OH C) CH3 CH3 CH3COH CH3 D) CH3CH2CH2CH2OH E) CH3CH2CH2OCH3 Ans: A Topic: Functional groups Section: 2.6 Difficulty Level: Easy 31. What functional group is present in the following compound? HO A) B) C) D) E) 1o alcohol ether 2o alcohol ester 3o alcohol Ans: E Topic: Functional groups Section: 2.6 Difficulty Level: Medium 32. Which compound is a tertiary alcohol? CH3 OH H3CH2C O HO CH3 I II III O IV A) B) C) D) E) I II III IV V Ans: E Topic: Functional groups Section: 2.1 and 2.6 Difficulty Level: Easy OH V 33. What functional group(s) is/are present in the following compound? OH A) B) C) D) E) alkyne and 2o alcohol alkyne and 1o alcohol 2o alcohol and alkene nitrile and 1o alcohol alkene and 2o alcohol Ans: A Topic: Functional groups Section: 2.5 and 2.6 Difficulty Level: Easy 34. A tertiary carbon atom is present in which of these compounds? Cl I Cl II III OH HO IV A) B) C) D) E) I II, IV III, V IV All of these Ans: C Topic: Functional groups Section: 2.5 and 2.6 Difficulty Level: Easy V 35. What functional group(s) is/are present in the following compound? Cl OH A) B) C) D) E) 1o alcohol and 2o alkyl chloride ether and 2o alcohol 1o alkyl chloride and 1o alcohol 1o alkyl chloride and 2o alcohol None of these Ans: D Topic: Functional groups, Isomerism Section: 1.3A, 2.6, and 2.7 Difficulty Level: Easy 36. A) B) C) D) E) How many constitutional isomers are possible with the formula C4H10O? 3 4 5 6 7 Ans: E Topic: Functional groups Section: 2.8 Difficulty Level: Easy 37. Which compound is a secondary amine? A) CH3CH2CH2NH2 B) CH3CHCH3 NH2 C) CH3CH2NH CH3 H C N CH3 D) 3 CH3 E) CH3CH2CHNH2 CH3 Ans: C Topic: Functional groups Section: 2.8 Difficulty Level: Easy 38. Which compound is a primary amine with the formula C5H13N? H2N NH H2N I II III N IV A) B) C) D) E) NH2 V I II III IV V Ans: C Topic: Functional groups Section: 2.8 Difficulty Level: Easy 39. What functional group is present in the following compound? N H A) B) C) D) E) 1o alkyl bromide 2o amine nitrile 1o amine 3o amine Ans: B Topic: Functional groups Section: 2.8 Difficulty Level: Easy 40. What functional group is present in the following compound? N A) B) C) D) E) 1o alkyl bromide 2o amine nitrile 1o amine 3o amine Ans: E Topic: Functional groups Section: 2.8 Difficulty Level: Easy 41. Which is a 3 amine? NH2 H2N NH I II III NH2 O N IV A) B) C) D) E) I II III IV V Ans: D Topic: Functional groups Section: 2.8 Difficulty Level: Easy V 42. An example of a tertiary amine is: H N NH2 I II NH2 IV A) B) C) D) E) H2N III N V I II III IV V Ans: E Topic: Functional groups Section: 2.7 and 2.8 Difficulty Level: Easy 43. What functional group(s) is/are present in the following compound? NCH3 O A) B) C) D) E) ether and 2o amine ester and 3o amine 3o amine 3o amine and ether None of these Ans: D Topic: Functional groups Section: 2.9 Difficulty Level: Easy 44. Which compound is a ketone? A) O C OH H O B) C) CH3CCH2CH3 O HCOCH3 D) O C H H E) H3C CH OH H 3C Ans: B Topic: Functional groups Section: 2.9 Difficulty Level: Easy 45. Which compound is an aldehyde? O O O NH O I II III O OH A) B) C) D) E) IV I II III IV V Ans: D V Topic: Functional groups Section: 2.9 Difficulty Level: Medium 46. What functional group is present in the following compound? CHO A) B) C) D) E) alcohol ketone aldehyde ester ether Ans: C Topic: Functional groups Section: 2.9 Difficulty Level: Medium 47. What functional group is present in the following compound? COCH3 A) B) C) D) E) alcohol ketone aldehyde ester ether Ans: B Topic: Functional groups Section: 2.1 and 2.9 Difficulty Level: Medium 48. What functional group(s) is/are present in the following compound? OHC A) B) C) D) E) Ketone and alkene Ketone and alkyne Aldehyde and alkene Aldehyde and alkyne 1o alcohol and alkene Ans: C Topic: Functional groups Section: 2.1, 2.6, and 2.9 Difficulty Level: Easy 49. The compound below is an adrenocortical hormone called cortisone. Which functional group is not present in cortisone? OH O OH O O A) B) C) D) E) 1 alcohol Ketone 3 alcohol Carboxylic acid Alkene Ans: D Topic: Functional groups Section: 2.1, 2.6, and 2.9 Difficulty Level: Easy 50. Which functional groups are present in the following compound? OH O H A) B) C) D) E) Alkene, 1º alcohol, ketone Alkene, 2º alcohol, aldehyde Alkene, 2º alcohol, ketone Alkyne, 1º alcohol, aldehyde Alkyne, 2º alcohol, ketone Ans: B Topic: Functional groups Section: 2.1, 2.6, and 2.9 Difficulty Level: Easy 51. The compound shown below is the male sex hormone, testosterone. OH O O A) B) C) D) E) In addition to a cycloalkane skeleton, testosterone also contains the following functional groups: Alkene, ester, tertiary alcohol. Alkene, ether, secondary alcohol. Alkene, ketone, secondary alcohol. Alkyne, ketone, secondary alcohol. Alkene, ketone, tertiary alcohol. Ans: C Topic: Functional groups Section: 2.1, 2.6, and 2.9 Difficulty Level: Medium 52. The compound shown below is a synthetic estrogen. It is marketed as an oral contraceptive under the name Enovid. OH O A) B) C) D) E) In addition to an alkane (actually cycloalkane) skeleton, the Enovid molecule also contains the following functional groups: Ether, alcohol, alkyne. Aldehyde, alkene, alkyne, alcohol. Alcohol, carboxylic acid, alkene, alkyne. Ketone, alkene, alcohol, alkyne. Amine, alkene, ether, alkyne. Ans: D Topic: Functional Groups Section: 2.7 and 2.9 Difficulty Level: Easy 53. Many organic compounds contain more than one functional group. Which of the following is/are both an aldehyde and an ether? O O O O O I O II III O O O OCH3 O V IV A) B) C) D) E) I II, IV V I, V III Ans: A Topic: Functional groups Section: 2.10A Difficulty Level: Easy 54. Which is a carboxylic acid? OH O OH O O I O II III O O O OH IV A) B) C) D) E) I II III IV V Ans: E Topic: Functional groups Section: 2.10A Difficulty Level: Medium V 55. What functional group(s) is/are present in the following compound? CO2H A) B) C) D) E) 1o alcohol and ketone carboxylic acid ester 1o alcohol and aldehyde alcohol Ans: B Topic: Functional groups Section: 2.10A Difficulty Level: Medium 56. What functional group(s) is/are present in the following compound? HO2C A) B) C) D) E) 1o alcohol and ketone ester carboxylic acid 1o alcohol and aldehyde alcohol Ans: C Topic: Functional groups Section: 2.1, 2.6, 2.9, and 2.10A Difficulty Level: Medium 57. Which functional group is not contained in prostaglandin E1? O H O HO H A) B) C) D) E) H OH H OH Prostaglandin E1 Ketone 2 alcohol 3 alcohol Carboxylic acid Alkene Ans: C Topic: Functional groups Section: 2.10B Difficulty Level: Easy 58. What functional group(s) is/are present in the following compound? O O A) B) C) D) E) ether and ketone carbonyl and ether carboxylic acid and ether ester 1o alcohol Ans: D Topic: Functional groups Section: 2.10B Difficulty Level: Easy 59. Which compound is an ester? O O O NH O I II III O OH IV A) B) C) D) E) V I II III IV V Ans: C Topic: Functional groups Section: 2.10B Difficulty Level: Medium 60. What functional group is present in the following compound? CO2CH3 A) B) C) D) E) alcohol ketone aldehyde ester ether Ans: D Topic: Functional groups Section: 2.6 and 2.10B Difficulty Level: Medium 61. What functional group(s) is/are present in the following compound? CO2CH2CH3 OH A) B) C) D) E) Ketone and 1o alcohol Ether and alcohol Ester and ether Ester and 1o alcohol 1o alcohol and aldehyde Ans: D Topic: Functional groups Section: 2.9 and 2.10B Difficulty Level: Medium 62. Which compound can be classified as an ester as well as a ketone? O O O O O O I II O III O O O O IV A) B) C) D) E) OH OH I II III IV V Ans: D Topic: Functional groups Section: 2.1, 2.6, 2.8, and 2.10B Difficulty Level: Hard V 63. Drawn below is Atropine, found in Atropa belladonna, sometimes used in dilating pupils during an eye-exam. Which of the following functional groups is NOT in atropine? N O O OH Atropine A) B) C) D) E) Amine Ester Alcohol Benzene Ring Ketone Ans: E Topic: Functional groups Section: 2.6 and 2.10C Difficulty Level: Medium 64. What functional group(s) is/are present in the following compound? HO O NHCH3 A) B) C) D) E) o 1 alcohol and 2o amine amide and 2o alcohol nitrile and 1o alcohol 2o amide and ether None of these Ans: E Topic: Functional groups Section: 2.1, 2.6, 2.7, and 2.10C Difficulty Level: Medium 65. The compound shown below is a substance called Capsaicin, found in varying concentrations in several varieties of hot peppers, and responsible for their respective degrees of “heat”. Which functional groups are present in the molecule of capsaicin? O N H O OH A) B) C) D) E) Capsaicin Alkene, ketone, amine, alcohol, ester Alkene, ketone, alcohol, ether Alkene, amine, phenol, ether Ether, phenol, alkene, amide Ester, phenol, alkene, amide Ans: D Topic: Functional groups Section: 2.8 and 2.11 Difficulty Level: Medium 66. What functional group(s) is/are present in the following compound? NC A) B) C) D) E) NHCH3 o 1 amine and 2o amine amide and 2o amine 2o amine and nitrile nitrile and 1o amine amide and nitrile Ans: C Topic: Intermolecular forces Section: 2.13 Difficulty Level: Easy 67. A) B) C) D) E) The strongest of attractive forces is which type? Dispersion forces Ion-dipole Dipole-dipole Cation-anion Hydrogen bonds Ans: D Topic: Intermolecular forces Section: 2.13 Difficulty Level: Easy 68. A) B) C) D) E) Which of these is the weakest of the intermolecular attractive forces? Ion-ion Dispersion forces Dipole-dipole Covalent bonding Hydrogen bonding Ans: B Topic: Intermolecular forces Section: 2.13 Difficulty Level: Medium 69. A) B) C) D) E) Which compound would you expect to have the highest melting point? n-Butyl alcohol Isobutyl alcohol sec-Butyl alcohol tert-Butyl alcohol Diethyl ether Ans: D Topic: Intermolecular forces Section: 2.13 Difficulty Level: Medium 70. Which of the following is not found in the following substance? CH3CH2CH2CH2CH2OH A) Ion-ion B) Dispersion forces C) Dipole-dipole D) Covalent bonding E) Hydrogen bonding Ans: A Topic: Intermolecular forces Section: 2.13 Difficulty Level: Medium 71. Which alkane is predicted to have the highest melting point of those shown? A) CH3CH2CH2CH3 B) CH3CHCH3 CH3 C) CH3CH2CH2CH2CH3 D) CH3CHCH2CH3 E) CH3 CH3 CH3CCH3 CH3 Ans: E Topic: Intermolecular forces Section: 2.13B Difficulty Level: Easy 72. A) B) C) D) E) What intermolecular forces hold base pairs together in DNA? Ion-ion Dipole-dipole Hydrogen bonds Dispersion forces Covalent bonds Ans: C Topic: Intermolecular forces Section: 2.13C Difficulty Level: Easy 73. Which compound would you expect to have the lowest boiling point? NH2 A) B) NH2 C) H N D) N E) NH2 Ans: D Topic: Intermolecular forces Section: 2.13C Difficulty Level: Easy 74. A) B) C) D) Which of these compounds would have the highest boiling point? CH3OCH2CH2CH2OCH3 CH3CH2OCH2CH2OCH3 CH3CH2OCH2OCH2CH3 CH3OCH2CHOCH3 CH3 E) HOCH2CH2CH2CH2CH2OH Ans: E Topic: Intermolecular forces Section: 2.13C Difficulty Level: Easy 75. A) B) C) D) E) Which compound would have the highest boiling point? CH3CH2CH2CH2CH2CH3 CH3CH2OCH2CH2CH3 CH3CH2CH2CH2CH2OH CH3CH2OCH(CH3)2 CH3OCH2CH2CH2CH3 Ans: C Topic: Intermolecular forces Section: 2.13C Difficulty Level: Easy 76. A) B) C) Of the following compounds, the one with the highest boiling point is: CH3CH3 CH3CH2Cl CH3C=O H D) CH3CH2OH E) CH3CH2OCH2CH3 Ans: D Topic: Intermolecular forces Section: 2.13C Difficulty Level: Medium 77. A) B) C) D) E) Which compound would you expect to have the highest boiling point? ethane ethene ethyne bromoethane methane Ans: D Topic: Intermolecular forces Section: 2.13C Difficulty Level: Medium 78. A) B) C) D) E) Which compound would you expect to have the highest boiling point? ethyl alcohol ethyl amine chloroethane water ethane Ans: D Topic: Intermolecular forces Section: 2.13C Difficulty Level: Medium 79. Which of these would you expect to have the lowest boiling point? A) CH3CH2CH2OH B) CH3CHCH3 OH C) CH3OCH2CH3 D) CH3CH2CH2CH2OH E) CH3CH2OCH2CH3 Ans: C Topic: Intermolecular forces Section: 2.13C Difficulty Level: Medium 80. Which compound would you expect to have the lowest boiling point? O A) NH2 H N B) O O C) N D) NH2 O E) O N H Ans: C Topic: Intermolecular forces Section: 2.13C Difficulty Level: Medium 81. A) B) C) D) E) Which compound would you expect to have the highest boiling point? CH3OCH2CH2OCH3 CH3OCH2OCH2CH3 HOCH2CH2CH2CH2OH CH3OCH2CH2CH2OH (CH3O)2CHCH3 Ans: C Topic: Intermolecular forces Section: 2.13C Difficulty Level: Medium 82. Which compound would have the lowest boiling point? OH OH O I II III OH OH IV A) B) C) D) E) V I II III IV V Ans: A Topic: Intermolecular forces Section: 2.13D Difficulty Level: Easy 83. The solid alkane CH3(CH2)18CH3 is expected to exhibit the greatest solubility in which of the following solvents? A) CCl4 B) CH3OH C) H2O D) CH3NH2 E) HOCH2CH2OH Ans: A Topic: Intermolecular forces Section: 2.13D Difficulty Level: Easy 84. The following substance is expected to have low solubility in which of the following solvent(s)? O Na O A) B) C) D) E) CCl4 C2H5OH CHCl3 CH2OHCH2CH2CH2CH2CH2OH The given substance is likely to be quite soluble in all of the solvents described. Ans: A Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Easy 85. For the functional group(s) on the following molecule what characteristic IR absorption(s) would be expected (ignoring C-H absorptions)? CHO A) B) C) D) E) A peak around 1700 cm-1 A peak around 3300 cm-1 Only normal alkane absorptions A peak around 2250 cm-1 None of these Ans: A Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Easy 86. For the functional group(s) on the following molecule what characteristic IR absorption(s) would be expected (ignoring C-H absorptions)? A) B) C) D) E) A peak around 1700 cm-1 A peak around 3300 cm-1 A peak around 1650 cm-1 A peak around 2250 cm-1 None of these Ans: C Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Easy 87. The IR spectrum of which type of compound will not show evidence of hydrogen bonding? A) Aldehyde B) Alcohol C) Carboxylic acid D) Phenol E) Primary amine Ans: A Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Easy 88. The IR spectrum of which type of compound generally exhibits evidence of hydrogen bonding? A) Aldehyde B) Carboxylic acid C) Alkene D) Ester E) Ketone Ans: B Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Easy 89. IR evidence for the presence of the C=C would be most difficult to detect in the case of which of these alkenes? A) B) C) D) E) Ans: D Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Medium 90. An oxygen-containing compound shows strong IR absorption at 1630-1780 cm-1 and 3200-3550 cm-1. What type of compound is it likely to be? A) An alcohol B) A carboxylic acid C) An ether D) A ketone E) An aldehyde Ans: B Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Medium 91. For the functional group(s) on the following molecule what characteristic IR absorption(s) would be expected (ignoring C-H absorptions)? CN A) B) C) D) E) A peak around 1700 cm-1 A peak around 3300 cm-1 A peak around 1650 cm-1 A peak around 2250 cm-1 None of these Ans: D Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Medium 92. For the functional group(s) on the following molecule, what characteristic IR absorption(s) would be expected (ignoring C-H absorptions)? HO A) B) C) D) E) Peaks around 1700 and 1650 cm-1 Peaks around 3300 and 1710 cm-1 Peaks around 1650 and 3300 cm-1 Only a peak around 3300 cm-1 None of these Ans: C Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Medium 93. For the functional group(s) on the following molecule, what characteristic IR absorption(s) would be expected (ignoring C-H absorptions)? CO2H A) B) C) D) E) Peaks around 1700 and 1650 cm-1 A strong broad peak over 3600 to 2500 and around 1710 cm-1 Peaks around 1650 and 3300 cm-1 Peaks around 3300 and 1710 cm-1 None of these Ans: B Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Medium 94. For the functional group(s) on the following molecule, what characteristic IR absorption(s) would be expected (ignoring C-H absorptions)? Br A) B) C) D) E) Peaks around 1710 and 1650 cm-1 A strong broad peak over 3600 to 2500 cm-1 Peaks around 1650 and 3300 cm-1 A peak around 1710 cm-1 None of these Ans: E Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Medium 95. For the functional group(s) on the following molecule, what characteristic IR absorption(s) would be expected (ignoring C-H absorptions)? A) B) C) D) E) Peaks around 3300, 2150, and 1650 cm-1 Peaks around 1710 and 1650 cm-1 Peaks around 1650 and 3300 cm-1 A peak around 2250 and 3300 cm-1 None of these Ans: A Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Medium 96. The absorption band for the O-H stretch in the IR spectrum of an alcohol is sharp and narrow in the case of: A) a Nujol mull of the alcohol. B) a concentrated solution of the alcohol. C) a gas phase spectrum of the alcohol. D) the spectrum of the neat liquid. E) none of these Ans: C Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Medium 97. A split peak for the IR absorption due to bond stretching is observed for the carbonyl group in which of these compounds? O A) CH3CH2CH2COH O B) ca C) CH3CH2CCl O CH3CH2CNH2 O D) CH3CH2COCH2CH3 O O E) CH3CH2COCCH2CH3 Ans: E Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Medium 98. The IR spectrum of which of the following substances is likely to show a small, but sharp peak at 2200 cm-1? OH O N H II I IV A) B) C) D) E) I II III IV V Ans: E Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Medium III V 99. A) B) C) D) E) An anticipated IR absorption band may not be observed because: it occurs outside the range of the instrument used. no change occurs in the dipole moment during the vibration. the absorption band is eclipsed by another. the intensity is so weak that it cannot be differentiated from instrument noise. All of these Ans: E Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Hard 100. For the functional group(s) on the following molecule what characteristic IR absorption(s) would be expected (ignoring C-H absorptions)? O O A) B) C) D) E) peaks around 1740 and 1650 cm-1 A strong broad peak over 3600 to 2500 cm-1 peaks around 1650 and 3300 cm-1 a peak around 1740 cm-1 None of these Ans: D Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Hard 101. For the following reaction sequence (it is not necessary to understand the chemistry) what significant change(s) would be expected by IR (ignoring C-H absorptions)? a. NaNH 2 b. CH 3CH 2I A) B) C) D) E) A peak around 1710 cm-1 would disappear. A peak around 1710 cm-1 would appear. A peak around 2150 cm-1 would disappear. No change would be observed. None of these Ans: C Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Hard 102. For the following reaction sequence (it is not necessary to understand the chemistry) what significant change(s) would be expected by IR (ignoring C-H absorptions)? O a. CH3MgBr OH b. H3O+ A) A peak around 1710 cm-1 would disappear and a new peak around 3300-3500 cm-1 would appear. B) A peak around 1710 cm-1 would appear and a new peak around 1650 cm-1 would disappear. C) A peak around 2150 cm-1 would disappear and a new peak around 3300-3500 cm-1 would appear. D) No change would be observed. E) None of these Ans: A Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Hard 103. For the following reaction sequence (it is not necessary to understand the chemistry) what significant change(s) would be expected by IR (ignoring C-H absorptions)? OH a. NaH OCH3 b. CH3I A) A peak around 3300 cm-1 would disappear . B) A peak around 1710 cm-1 would appear and a new peak around 3300 cm-1 would disappear. C) A peak around 2150 cm-1 would disappear and a new peak around 3300 cm-1 would appear. D) No change would be observed. E) None of these Ans: A Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Hard 104. For the following reaction sequence (it is not necessary to understand the chemistry) what significant change(s) would be expected by IR (ignoring C-H absorptions)? OH PCC O A) A peak around 3300 cm-1 would disappear and nothing new would appear. B) A peak around 1710 cm-1 would appear and a new peak around 3300 cm-1 would disappear. C) A peak around 2150 cm-1 would disappear and a new peak around 3300 cm-1 would appear. D) No change would be observed. E) None of these Ans: B Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Hard 105. For the following reaction sequence (it is not necessary to understand the chemistry) what significant change(s) would be expected by IR (ignoring C-H absorptions)? H2, Pd/C A) A peak around 3300 cm-1 would disappear and nothing new would appear B) A peak around 1710 cm-1 would appear and a new peak around 3300 cm-1 would disappear. C) A peak around 1650 cm-1 would disappear and nothing new would appear. D) No change would be observed. E) None of these Ans: C Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Hard 106. For the following reaction sequence (it is not necessary to understand the chemistry) what significant overall change(s) would be expected by IR (ignoring C-H absorptions)? 1. Br2, light 2. Zn, HCl A) A peak around 3300 cm-1 would disappear and nothing new would appear. B) A peak around 1710 cm-1 would appear and a new peak around 3300 cm-1 would disappear. C) A peak around 1650 cm-1 would disappear and nothing new would appear. D) No overall change would be observed. E) None of these Ans: D Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Hard 107. For the following reaction sequence (it is not necessary to understand the chemistry) what significant change(s) would be expected by IR (ignoring C-H absorptions)? CN a. DiBAl-H CHO b. H3O+ A) A peak around 2250 cm-1 would disappear and nothing new would appear. B) A peak around 1720 cm-1 would appear and a new peak around 3300 cm-1 would disappear. C) A peak around 2250 cm-1 would disappear and new peak around 1720 cm-1 would appear. D) A peak around 2250 cm-1 would disappear and new peak around 3300 cm-1 would appear. E) No change would be observed. Ans: C Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Hard 108. A) B) C) D) E) The IR stretching frequency occurs at the lowest frequency for which of these bonds? C–H C–O C–Br C–N C–F Ans: C Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Hard 109. The IR stretching frequency can be predicted to occur at the highest frequency for which of these bonds? A) C–H B) C–F C) C–Cl D) C–Br E) C–I Ans: A Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Hard 110. The IR absorption due to the stretching of which of these carbon-hydrogen bonds occurs at the highest frequency? H H H I II III H IV A) B) C) D) E) I II III IV V Ans: E Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Hard H V 111. An oxygen-containing compound which shows sharp IR absorption at 2200 cm-1 and 3300 cm-1 is likely to be what type of compound? A) An ester B) An alkene C) An alkyne D) An ether E) An aldehyde Ans: C SHORT ANSWER QUESTIONS Topic: Functional Groups Section: 2.1 Difficulty Level: Easy 112. Hydrocarbons containing carbon-carbon double bonds are referred to as ___________. Ans: alkenes Topic: Functional Groups Section: 2.1 Difficulty Level: Easy 113. Unsaturated hydrocarbons may be distinguished from saturated hydrocarbons by the presence of one or more _____________. Ans: Pi bonds Topic: Isomers, Functional Groups Section: 2.1 Difficulty Level: Easy 114. Draw a structural formula for C8H18 , in which there are two quaternary carbons. Ans: Topic: General Section: 2.1 Difficulty Level: Easy 115. The six p-electrons in benzene are _____________ about the ring, which explains why all of the C-C bonds are the same length. Ans: delocalized Topic: Isomers, Functional Groups Section: 1.3A and 2.1 Difficulty Level: Easy 116. Draw all isomers of C6H14. Ans: Topic: General Section: 2.2 Difficulty Level: Easy 117. A polar covalent bond is one in which electrons are _____________. Ans: not shared equally Topic: Molecular Geometry, Dipole Moment Section: 2.2 and 2.3 Difficulty Level: Medium 118. Carbon dioxide is non-polar, despite the fact that oxygen is much more electronegative than carbon. Briefly explain why, using relevant diagrams as appropriate to illustrate your Answer. Ans: The overall dipole moment of a polyatomic molecule depends on two factors: the polarity of various bonds and molecular geometry, since dipole forces have both magnitude and direction. In some molecules containing bonds of identical polarity, the molecular geometry may result in a net cancellation of the overall dipole forces. This is what happens in carbon dioxide: although there are two polar C-O bonds, because of the linear geometry of the molecule, the net dipole is zero. .. .. : O C O: Topic: Functional Groups Section: 2.4 Difficulty Level: Easy 119. Organic compounds are classified into chemical families on the basis of similarities in chemical properties; these similarities are primarily due to the presence of characteristic arrangements of atoms known as ________________. Ans: functional groups Topic: Isomers, Functional Groups Section: 1.3A, 2.6, and 2.7 Difficulty Level: Easy 120. Draw all isomers of C3H8O and classify each according to functional group. OH Ans: C3H8O OH O primary alcohol secondary alcohol ether Topic: Isomers, Functional Groups Section: 1.3A and 2.8 Difficulty Level: Medium 121. Draw all tertiary amine isomers of C6H15N. Ans: N N N N N N Topic: Functional Groups Section: 2.9 Difficulty Level: Easy 122. A group in which a carbon atom has a double bond to an oxygen atom is called a __________. Ans: carbonyl Topic: Isomers, Functional Groups Section: 2.9 Difficulty Level: Easy 123. Draw all isomers of C6H12O that are aldehydes and contain at least one secondary carbon. Ans: O O O O Topic: Isomers, Functional Groups Section: 1.3A and 2.9 Difficulty Level: Easy 124. Draw all isomers of C6H12O that are aldehydes. Ans: O O O O O O O Topic: Isomers, Functional Groups Section: 1.3A and 2.9 Difficulty Level: Easy 125. Draw all isomers of C5H10O that are ketones. O O O Ans: Topic: Intermolecular Forces Section: 2.13C Difficulty Level: Easy 126. Ethanol, C2H5OH, and propane, C3H8, have approximately the same molar mass, yet ethanol has a much higher boiling point. Briefly explain why. Ans: Strong hydrogen bonding between molecules of ethanol leads to elevation in boiling point. No hydrogen bonding is possible between molecules of propane, resulting in a lower boiling point compared with ethanol. Topic: Intermolecular Forces Section: 2.13C Difficulty Level: Easy 127. Ethanol, C2H5OH, and dimethyl ether, CH3OCH3, have the same molar mass, yet ethanol has a much higher boiling point. Briefly explain why. Ans: Strong hydrogen bonding between molecules of ethanol leads to elevation in boiling point. No hydrogen bonding is possible between molecules of dimethyl ether, resulting in a lower boiling point compared with ethanol. Topic: Bonding, Solubility Section: 2.13 specifically 2.13D Difficulty Level: Medium 128. Sodium chloride, which is quite soluble in water, is not very soluble in hexane. Why? Ans: Sodium chloride, which is an ionic substance, is soluble in a polar solvent such as water, but not in a non-polar solvent such as hexane. Topic: Functional Groups, IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Medium 129. Examine the following IR spectrum, for substance P (C8H22O). Which oxygen containing functional group is present in P? Ans: An aldehyde Topic: Functional Groups, IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Medium 130. Examine the following IR spectrum, for substance P (C5H12O). Which oxygen containing functional group is present in P? Ans: Alcohol Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Hard 131. The IR absorption frequencies of the C-H bond in alkanes, alkenes and alkynes are measurably different. Briefly explain why. Ans: IR absorption frequency depends on bond strength; the bond strength of C-H bonds in alkanes, alkenes and alkynes is different because different atomic orbitals (hybridized) of carbon are involved in the bond: the C-H bond in alkanes is described as (sp3-s), that in alkenes is (sp2-s) and in alkynes, it is (sp-s). The relative % s v. % p character of the hybrid orbitals of carbon would indicate different bond lengths / bond strengths for alkanes, alkenes and alkynes, with the bond length / bond strength being the longest/weakest respectively. This results in different IR absorption frequencies. Topic: IR Spectroscopy Section: 2.15 and 2.16 Difficulty Level: Medium 132. IR absorption signals of alcohols are typically broad. However, IR spectra of gaseous samples show sharp peaks. Briefly explain why. Ans: Broad signals of alcohols are due to hydrogen bonding associated with the O-H group. In gaseous samples, no hydrogen bonding is possible, and the signal becomes sharp. Topic: Functional Groups, IR Spectroscopy Section: 2.1, 2.15, and 2.16 Difficulty Level: Easy 133. An IR spectrum has significant peaks at 3080 and 1650 cm-1. What functional group is present in the molecule? Ans: An alkene Topic: Functional Groups, IR Spectroscopy Section: 2.1, 2.15, and 2.16 Difficulty Level: Hard 134. An IR spectrum has significant peaks at 2200 and 3300 cm-1. What functional group is present in the molecule? Ans: A terminal alkyne






