Bunsen Burner Beginnings
advertisement

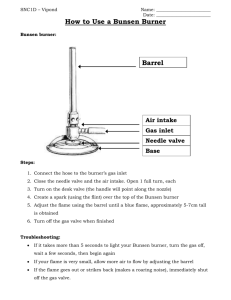
NAME:___________________________________ DATE:_____________________ PERIOD:_____ #:_____ Bunsen Burner Beginnings Lab BACKGROUD Chemistry is a basic science that deals with matter and energy relationships, the composition of matter, and the change matter undergoes. Chemistry uses specific scientific methods to solve problems. Scientific methods are organized, systematic ways to determine answers through experimentation. Observation, investigation, analysis, and interpretation are among the most important of all scientific methods. To investigate the properties of matter, we use our senses and devices available in the laboratory. Careful observations are made and recorded, then analyzed and interpreted. Our interpretation of the observations is connected to past experience. The final conclusions can be used to formulate theories. This laboratory is intended to help you become familiar with some of the scientific tools that a chemist uses to solve problems. A Bunsen burner will be observed. A comparison will be made between a luminous and nonluminous flame. In the process of the investigation, you will also discover the products of a combustion reaction. Observations should be organized and recorded so that they can be analyzed carefully before interpretations are made. SAFETY Wear safety goggles and an apron. It is important that you and your partners conduct all experiments by using good safety techniques. Read all safety precautions in your procedures and discuss them with your teacher EQUIPMENT and MATERIALS 1. Goggles 2. Apron 3. Evaporating Dish 4. Tongs 5. Bunsen Burner 6. Flint Striker (Sparker) 7. Wire Gauze PROCEDURE Part A: Identification of the parts of a Bunsen burner. While the details of construction vary among burners, each has a gas inlet located in the base, and a vertical tube or barrel in which the gas is mixed with air. There are adjustable openings or ports in the base of the barrel. These ports admit air to the gas stream and can be adjusted by rotating the collar at the base of the barrel. The burner that you will be using has an adjustable needle valve to regulate the flow of gas. The burner is always turned off at the gas valve, never the needle valve. COMPLETE PART A OF THE LAB WRITE-UP. 8. 2cm Sample of Copper Wire 9. 2cm Sample of Iron Wire 10. 2cm Sample of Aluminum Wire Bunsen Burner Beginnings Lab SAFETY When using an open flame, confine long hair and loose clothing. Long hair is tied in back of the head and away from the front of the face. Long sleeves on shirts, blouses, and sweaters are rolled up away from the wrists. You should also know the locations of fire extinguishers, fire blankets, safety showers and how to use them in case of a fire. Part B: Lighting & adjusting the Bunsen burner. Your teacher will demonstrate the correct use and lighting of the Bunsen burner. You are to follow these instructions EXACTLY as stated. When the best adjustment is reached, two distinct cones are visible in the flame. LIGHTING FLAME: 1. Clear the area of all flammable objects (paper, etc.). 2. Rotate the barrel until the air intake openings are completely closed. Then, turn barrel three full turns to open air intakes. 3. Close the gas flow valve at the bottom of the burner. Then, turn valve three full turns. 4. Connect the hose to the gas valve on the lab bench. 5. Turn on the gas valve by turning it parallel with the hose. 6. Use the striker to light the burner. ADJUSTING FLAME: 1. If the flame is too large, decrease the flow of gas to the burner by turning valve at base of burner. 2. If the flame is too yellow, increase the air supply by opening the air intakes valves more. 3. If the flame disappears down the burner barrel, turn the burner off at the gas valve, decrease the air supply, and try to light the burner again 4. A loud roaring sound indicates the presence of too much air, decrease the air flow by closing the air intake valves more. TURNING OFF FLAME: 1. When the flame is not being used, turn the burner off at the gas outlet valve. 2. NEVER turn off the burner at the needle valve at the base of the burner. SAFETY NOTE: If a very low flame is needed, remember that the air holes should be partially closed when the gas pressure is reduced. Otherwise the flame may burn inside the barrel. When improperly burning in this way, the barrel will get very hot and the flame will produce a poisonous gas, carbon monoxide. CAUTION: Carbon monoxide is a poisonous gas. If the flame is burning inside the base of the barrel, immediately turn off the gas at the gas outlet. Do not touch the barrel of the burner, for it is extremely hot. Allow the barrel of the burner to cool off and then proceed as follows. Begin again, but first decrease the amount of air admitted to the burner by partly closing the ports. Turn the gas full on and then relight the burner. Control the height of the flame by adjusting the needle valve. By taking these steps, you should acquire a flame that is burning safely and is easily regulated throughout the experiment. COMPLETE PART B OF THE LAB WRITE-UP. AFTER INSTRUCTOR APPROVAL, START PART C. Bunsen Burner Beginnings Lab Part C. Observations of the Bunsen burner. 1. Light the Bunsen burner using the directions on the previous page. 2. Regulate the flow of gas to give a flame extending roughly 8 cm above the barrel. Now adjust the supply of air until you have a quiet steady flame with a sharply defined, light blue inner cone. Once you have a flame that is burning safely and steadily, draw a sketch of it in your lab sheet. Describe your observations of the non-luminous flame in your data table. 3. Using tongs, hold the underside of an evaporating dish in the tip of the flame for about three minutes. Place the inverted dish the wire gauze on the lab table. Allow the dish to cool and then examine its underside. Describe and explain the results after heating the evaporating dish for about 3 minutes in your data table. 4. To gain information about the relative temperature of different regions of the flame, use crucible tongs to hold a 2-cm strips of different types of metals into three regions of the flame. a. Hold a strip of copper in location A for 2 minutes. Record observations. b. Hold a second strip of copper in location B for 2 minutes. Record observations. c. Hold a third strip of copper in location C for 2 minutes. Record observations. d. Repeat steps a-c with aluminum wire and then iron wire. Record observations. 5. Close the air intake ports at the base of the burner until you have a bright, orange colored flame. Draw a sketch of this flame on your lab sheet. Describe your observations of the luminous flame in your data table. 6. Repeat steps 3 and 4 using the luminous flame. Positions A, B, and C of the luminous flame are seen to the right. 7. Extinguish the Bunsen burner flame using the gas control valve. Once completely cooled, clean and properly store all lab equipment. NAME:___________________________________ DATE:_____________________ PERIOD:_____ #:_____ Bunsen Burner Beginnings Lab LAB WRITE-UP PART A - Name the regions of the Bunsen burner and briefly describe their functions. REGION A B C D E F NAME FUNCTION Bunsen Burner Beginnings Lab PART B – Answer the following questions about lighting and adjusting the Bunsen burner. 1. 2. 3. 4. Flammable materials should be removed before lighting the flame. The gas is on when the gas valve is perpendicular to the hose. The air intake ports should be completely closed when lighting the burner. The gas flow valve should be opened three turns before lighting the burner. True / False True / False True / False True / False 5. What do you do if the flame is too large? 6. What do you do if the flame is too yellow? 7. Why is it dangerous for the flame to burn within the barrel? 8. What actions should be taken if the flame is burning within the barrel? 9. Describe the proper method for turning off a Bunsen burner. PART C – Record your observations when working with the non-luminous and luminous flames. 1. Sketch the two types of flames. Sketch of non-luminous flame: Sketch of luminous flame: Bunsen Burner Beginnings Lab 2. Fill in the table below. First fill in your observations of the non-luminous flame, and then your observations of the luminous flame. Type of Flame Non-Luminous Luminous Description Results of Heating Evaporating Dish A B C A B C A B C A B C A B C A B C Results of Heating Copper Wire Results of Heating Iron Wire Results of Heating Aluminum Wire Bunsen Burner Beginnings Lab CONCLUSIONS 1. What safety precautions should be taken before working with a Bunsen burner? 2. Which type of flame is cleaner? What data do you have to support this conclusion? 3. Which type of flame is hotter? What data do you have to support this conclusion? 4. The melting temperature of copper is 1,084ᵒC, the melting point of aluminum is 660ᵒC, and the melting point of iron is 1,535ᵒC. Use this information to answer the questions below. a. What can you conclude about the temperatures of the different regions of the nonluminous flame? b. What can you conclude about the temperatures of the different regions of the luminous flame? 5. Based on your analysis of the two flames, what type of flame is preferred for laboratory work? Provide two pieces of evidence to support your conclusion.