Metric Conversions Ladder Method
advertisement

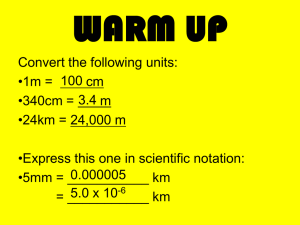
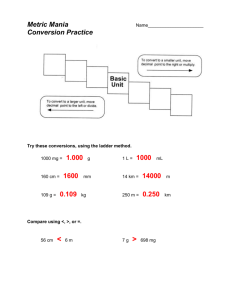
Metric Mania and Density Answer the following questions! You may use your notes from yesterday. • How many significant figures are Write the following in Scientific in each number? Notation. 1. 1.23 2. 0.0045 3. 0.100 4. 9.001 1. 70200 2. 5000 3. 0.00042 4. 0.00104 Write in standard form. 1. 2.5 x 107 2. 4.08 x 10-2 Metric Conversions Ladder Method T. Trimpe 2008 http://sciencespot.net/ How do you use the “ladder” method? 1st – Determine your starting point. 2nd – Count the “jumps” to your ending point. 3rd – Move the decimal the same number of jumps in the same direction. 4 km = _________ m Starting Point Ending Point How many jumps does it take? 4. __. 1 . = 4000 m . __ __ 2 3 Conversion Practice Try these conversions using the ladder method. 1000 mg = _______ g 1 L = _______ mL 160 cm = _______ mm 14 km = _______ m 109 g = _______ kg 250 m = _______ km Metric Conversion Challenge Write the correct abbreviation for each metric unit. Kilogram _____ Milliliter _____ Kilometer _____ Meter _____ Millimeter _____ Centimeter _____ Gram _____ Liter _____ Milligram _____ Try these conversions, using the ladder method. 480 cm = _____ m 5.6 kg = _____ g 8 mm = _____ cm 5.6 m = _____ cm 120 mg = _____ g Use your notes from yesterday and answer the following: Compare using <, >, or =. 25) 63 cm 27) 5 g 6m 508 mg 29) 1,500 mL 1.5 L Find your name in purple ink and sit at that table. Organization Notebooks • You should have a 3 ring binder for class with the following tabs • Notes • Classwork/ homework • Labs • Quizzes/Tests/Review Sheets Lab Composition Notebook • The first page front and back will be for the table of contents. • The following pages will need to have the following information at the top • Lab Title • Date DENSITY Volume The amount of space occupied by an object is called its volume. •V=lxwxh • Water Displacement • Final water level- initial water level= V Mass -is a measure of the quantity of matter in an object Density • Density is the mass per unit volume of a material. • You find density by dividing an object’s mass by the object’s volume. • D= M/V You have a sample with a mass of 620 g & a volume of 753 cm3. Find density. • G • U • E • S • S A liquid has a density of 0.87 g/mL. What volume is occupied by 25 g of the liquid? • G • U • E • S • S An object has a volume of 825 cm3 and a density of 13.6 g/cm3. Find its mass. • G • U • E • S • S Exit Ticket • 164 cm = _________ m • 25 mm= _________ km • 16g= _________ kg • Use the GUESS method • A piece of tin has a mass of 16.52 g and a volume of 2.26 cm3. What is the density of tin?