Periodic Table LDC 3D Periodic Trends Activity Instruction
advertisement

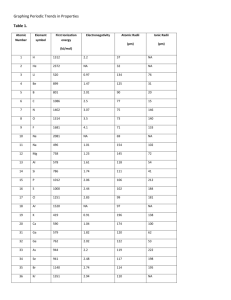
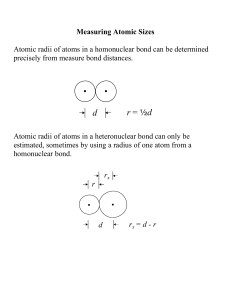
3-D Periodic Trend Model Activity Each group will create a three dimensional periodic table that satisfactorily illustrates the assigned periodic property of the elements. Instructions: Use the 96 well-plate as a base to support drinking straws. Determine a reasonable scale for the length of straws to correspond to the needed values: o atomic radii (1cm = 10pm) o ionic radii (1cm = 10pm) o ionization energy (1 cm = 100 kJ/mol; example 1312 kJ/mol = 13.1 cm) o electronegativity (1 inch = 1 electronegativity value) Cut the straws to the appropriate length for each element in Table I (Part I). Place straws in sequence in the well plate to create a 3-D graph of the trend. Use the illustrations below for placing the straws in the well plate: OOOOOOOOOOOO OOOOOOOOOOOO OOOOOOOOOOOO OOOOOOOOOOOO OOOOOOOOOOOO OOOOOOOOOOOO OOOOOOOOOOOO OOOOOOOOOOOO Scale __________ H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Ga Ge As Se Br Kr Table 1. Atomic Number 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 31 32 33 34 35 36 Element symbol H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Ga Ge As Se Br Kr First Ionization energy (kJ/mol) 1312 2372 520 899 801 1086 1402 1314 1681 2081 496 738 578 786 1012 1000 1251 1520 419 590 579 762 944 941 1140 1351 Electronegativity 2.2 NA 0.97 1.47 2.01 2.5 3.07 3.5 4.1 NA 1.01 1.23 1.61 1.74 2.06 2.44 2.83 NA 0.91 1.04 1.82 2.02 2.2 2.48 2.74 2.94 Atomic Radii (pm) 37 32 134 125 90 77 75 73 71 69 154 145 118 111 106 102 99 97 196 174 120 122 119 117 114 110 Ionic Radii (pm) NA NA 76 31 20 15 146 140 133 NA 102 72 54 41 212 184 181 NA 138 100 62 53 222 198 195 NA