Lesson 13 - Oregon State University
advertisement

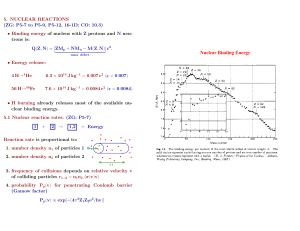
Lesson 13 Nuclear Astrophysics Elemental and Isotopic Abundances Elemental and Isotopic Abundances (cont.) Elemental and Isotopic Abundances Overview of the Sun and the Nucleosynthetic Processes Involved Primordial Nucleosynthesis • Age of the universe 10-20 billion years with best estimate being 14 1 x 109 y. • Universe started with the Big Bang. • Evidence for the Big Bang: 2.7 K microwave radiation. • Photon density ~ 400/cm3 Early history of the Universe Evolution of the Universe • 10-43 s Planck time 1032 K, vol=10-31 volcurrent • kBT(eV)=8.5x10-5 T(K) • Matter is QGP, all particle present. • 10-6 s, T ~1013K, hadronic matter condenses out. • Matter is nucleons, mesons, neutrinos, photons, electrons. Evolution of the Universe • 10-2 s. T~ 1011 K, ~ 4 x 106 kg/m3 1.5x1010 T (K ) t(s) e p e n e n p e Evolution of the Universe • At T=1012K, n/p ~ 1, at T=1011 K n/p ~ 0.86, etc. At T = 1011K, no complex nuclei were formed because the temperature was too high to allow deuterons to form. When the temperature fell to T= 1010K (t~ 1 s), the creation of e+/e- pairs (pair production) ceased because kT < 1.02 MeV and the neutron/proton ratio was ~ 17/83. At a time of 225s, this ratio was 13/87, the temperature was T ~ 109K, then density was ~ 2 x 104kg/m3, and the first nucleosynthetic reactions occurred. First Nucleosynthesis • Hydrogen burning • n+pd+ • p + d 3He + • n + d 3H + • He formation • 3H + p 4He + • 3He + n 4He + • 3H + d 4He + n • d + d 4He + Big Bang Nucleosynthesis After about 30 m, nucleosynthesis ceased. The temperature was ~ 3 x 108K and the density was ~ 30 kg/m3. Nuclear matter was 76% by mass protons, 24% alpha particles with traces of deuterium, 3He and 7Li. The /n/p ratio is 109/13/87. The relative ratio of p/4He/d/3He/7Li is a sensitive function of the baryon density of the Universe. Chemistry began about 106 years later, when the temperature had fallen to 2000K and the electrons and protons could combine to form atoms. Further nucleosynthesis continues to occur in the interiors of stars. Stellar Nucleosynthesis • All elements beyond H and He synthesized in stellar interiors • Stellar nucleosynthesis continues to date (2 x 105 y 99Tc lines in stars) Stellar Evolution • Population III stars (protostars)--H, He, short lifetimes, now extinct • Population II stars (H, He, 1% heavier elements) • Population I stars (H, He, 2-5% heavier elements) Includes our sun. Sun • • • • • • • Typical Population I star. mass=2 x 1030 kg radius=7 x 106 m ~1.41 x 103 kg/m3 surface T ~ 6000K Luminosity ~ 3.83 x 1026 W age ~ 4.5 x 109 y. Herzsprung-Russell Diagrams Stellar evolution and H-R diagrams Aside on our Sun • ~ 7 x 109 more years on main sequence • 1.1-1.5 x 109 years, luminosity will increase by ~10%, making Earth uninhabitable. • Terrestrial life has used up about 3/4 of its lifespan. Supernovas • • • • Massive stellar explosions ~1051 ergs released in a few seconds 2-3/century, last observation was 1987. Some supernovas lead to the formation of neutron stars. Thermonuclear reaction rates R N x N y (v)vdv N x N y v 0 N x N y v R 1 xy m P(v) 2kT 3/ 2 8 1/ 2 1 v 3/ 2 kT mv2 exp 2kT E (E)E exp kT dE 0 But these are charged particle reactions! •For p +p, CB ~ 550 keV. •kT~ 1.3 keVbarrier penetration problem P exp( 1/ 2 2Z1Z2 e 2 ) exp(31.29Z1Z2 ) E v 1/ 2 1 (E) exp S(E) 31.29Z1Z2 E E 8 1/ 2 1 v 3/ 2 kT E b dE 1/ 2 S(E)exp kT E 0 Stellar Nucleosynthesis--A Scorecard • Big Bang 75% H, 25% He, trace 7Li • From ~ 106 years after the Big Bang to present, get nuclear fusion reactions in stars that synthesize the elements up to A ~ 60. Fuel T(K) kT(MeV) H 5 x 107 0.002 He 2 x 108 0.02 12 C 8 x 108 0.07 16 O 2 x 109 0.2 Ne 1.5 x 10 9 0.13 3.5 x 10 9 0.3 1 4 20 28 Si Products 4 He 12 C, 16O, 20Ne 16 O, 20Ne, 24Mg 20 Ne, 28Si, 32S 16 O, 24Mg A < 60 Hydrogen Burning • • • • • First stage of a star; converts H into He. First reaction(pp): p + p d + e+ +e Q = 0.42 MeV Weak branch (pep) p + e- + p d + e Q = 1.42 MeV Next Reaction d + p 3He + Q = 5.49 MeV. 86% Branch 3He + 3He 4He + 2p Q = 12.96 MeV • Net reaction • 4p 4He + 2e+ + 2e Q = 26.7 MeV Hydrogen Burning(ppI chain) Side reaction • 3He + 4He 7Be + e • e- + 7Be 7Li + e Q = 0.86 MeV • p + 7Li 2 4He • This side branch along with the p+p, d+p is called the ppII process Another side branch • 7Be + p 8B + • 8B 8Be* + e+ + e • 8Be* 2 4He • This sequence along with the p+p, d+p, etc is called the PPIII chain. CNO Cycle + p 13N + 13N 13C + e+ + e 13C + p 14N + 14N +p 15O + 15O 15N + e+ + e 15N + p 12C + 4He 12C Net effect is 4p 4He + 2e+ + 2e CNO Cycle He burning • Eventually the H fuel will be exhausted, get gravitational collapse, further heating and red giant formation. Then He burning will commence. The reaction is the 3 process. 3 4He 12C Q = 7.37 MeV + 12C 20Ne + 4He 12C + 12C 23Na + p 12C + 12C 23Mg + n 12C + 12C 24Mg + 16O + 16O 24Mg + 2 4He 16O + 16O 28Si + 4He 16O + 16O 31P + p 16O + 16O 31S + n 16O + 16O 32S + 12C Reaction Time H burning 6 x 109 years He burning 0.5 x 10 6 years C burning 200 years Ne burning 1 year O burning Few months Si burning days Synthesis of A > 60 • Use neutron capture • There are two types of n-capture reactions. one on a slow time scale, the sprocess and one on a rapid time scale, the r process. • In s process reactions, - decay intervenes between n captures while in the r process, it does not. s-Process • Example • 56Fe + n 57Fe (stable) + • 57Fe + n 58Fe (stable) + • 58Fe + n 59Fe (t1/2 = 44.5 d) + • 59Fe 59Co (stable) + - + e •Process terminates at 209Bi 3(n, ) Bi(n, ) Bi Po Pb Pb 209Bi 209 210 210 206 209 r-process ( in supernovas) p process • makes proton-rich nuclei • most reactions are photonuclear reactions like (,p), (, n), (, ) • probably occurs in supernovas. rp process Solar Neutrinos Sun emits 1.8 x 1038 neutrinos/s with the flux hitting the Earth being 6.4 x 1010 neutrinos/s/cm2. Table 12.3 Predicted solar neutrino fluxes (Bahcall and Pena-Garay) Source Flux(particles/s/cm2) pp 5.94 x 10 10 pep 1.40 x 10 8 hep 7.88 x 10 3 7 Be 4.86 x 10 7 8 5.82 x 10 6 B 13 5.71 x 10 8 15 5.03 x 10 8 N O 17 F 5.91 x 10 6 Fluxes and Detectors Solar Neutrino Detectors • The most famous detector is the Chlorine detector of Ray Davis. • Contained 100,000 gal C2Cl4 in a cavern 1600 m below the earth’s surface in the Homestake kine. • Reaction used: Cl Ar e 37 37 e •Ar nuclei collected as gas, detect 2.8 keV e from Auger from EC Produce ~3 atoms/wk in volume of 1030 atoms. Gallex detector • e + 71Ga 71Ge + e• Collect Ge gas product • threshold = 0.232 MeV Super K • Detect Cerenkov radiation from • + e- + e• threshold = 8 MeV SNO • + e- + e• e + d 2p + e• +dn+p+ • Sensitive to all types of neutrinos Results • Davis : measured 2.1 +- 0.3 SNU expect 7.9 +- 2.4 SNU 1 SNU = 10-36 neutrino captures/s/target atom • Gallex: measured 77 +- 10 SNU expect 127 SNU Solution • neutrino oscillations • imply neutrino has mass Synthesis of Li, Be, B