Chapter 25
advertisement

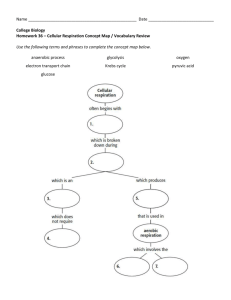
Chapter 25 Metabolism and Nutrition • The food we eat is our only source of energy for performing biological work. • There are three major metabolic destinations for the principle nutrients. They will be used for energy for active processes, synthesized into structural or functional molecules, or synthesized as fat or glycogen for later use as energy. Glucose Catabolism Coenzymes • Two coenzymes are commonly used by living cells to carry hydrogen atoms: nicotinamide adenine dinucleotide (NAD) and flavin adenine dinucleotide (FAD). • An important point to remember about oxidation-reduction reactions is that oxidation is usually an energy-releasing reaction. Mechanisms of ATP Generation • Phosphorylation is – bond attaching 3rd phosphate group contains stored energy • Mechanisms of phosphorylation – within animals • substrate-level phosphorylation in cytosol • oxidative phosphorylation in mitochondria – in chlorophyll-containing plants or bacteria • photophosphorylation. Phosphorylation in Animal Cells • In cytoplasm (1) • In mitochondria (2, 3 & 4) Carbohydrate Review • In GI tract – polysaccharides broken down into simple sugars – absorption of simple sugars (glucose, fructose & galactose) • In liver – fructose & galactose transformed into glucose – storage of glycogen (also in muscle) • In body cells --functions of glucose – oxidized to produce energy – conversion into something else – storage energy as triglyceride in fat Fate of Glucose • Glucose can be used to form amino acids, which then can be incorporated into proteins. • Excess glucose can be stored by the liver and skeletal muscles as glycogen, a process called glycogenesis. • If glycogen storage areas are filled up, liver cells and fat cells can convert glucose to glycerol and fatty acids that can be used for synthesis of triglycerides (neutral fats) in the process of lipogenesis. Glucose Movement into Cells • In GI tract and kidney tubules – Na+/glucose symporters • Most other cells – GluT facilitated diffusion transporters – insulin increases the insertion of GluT transporters in the membrane of most cells – in liver & brain, always lots of GluT transporters • Glucose 6-phosphate forms immediately inside cell (requires ATP) thus, glucose is “hidden” when it is in the cell. – Concentration gradient remains favorable for more glucose to enter. Glucose Catabolism Glucose Oxidation • Cellular respiration – 4 steps are involved – glucose + O2 produces H2O + energy + CO2 • Anaerobic respiration – called glycolysis (1) – formation of acetyl CoA (2) is transitional step to Krebs cycle • Aerobic respiration – Krebs cycle (3) and electron transport chain (4) Glycolysis • Glycolysis refers to the breakdown of the six-carbon molecule, glucose, into two three-carbon molecules of pyruvic acid. – 10 step process occurring in cell cytosol – use two ATP molecules, but produce four, a net gain of two (Figure 25.3). Glycolysis of Glucose & Fate of Pyruvic Acid • Breakdown of six-carbon glucose molecule into 2 three-carbon molecules of pyruvic acid – Pyruvic acid is converted to acetylCoA, which enters the Kreb’s Cycle. – The Kreb’s Cycle will require NAD+ • NAD+ will be reduced to the highenergy intermediate NADH. Glycolysis of Glucose & Fate of Pyruvic Acid When O2 falls short in a cell – pyruvic acid is reduced to lactic acid • coupled to oxidation of NADH to NAD+ • NAD+ is then available for further glycolysis – lactic acid rapidly diffuses out of cell to blood – liver cells remove lactic acid from blood & convert it back to pyruvic acid Pyruvic Acid • The fate of pyruvic acid depends on the availability of O2. Formation of Acetyl Coenzyme A • Pyruvic acid enters the mitochondria with help of transporter protein • Decarboxylation – pyruvate dehydrogenase converts 3 carbon pyruvic acid to 2 carbon fragment acetyl group plus CO2. Formation of Acetyl Coenzyme A • 2 carbon fragment (acetyl group) is attached to Coenzyme A to form Acetyl coenzyme A, which enter Krebs cycle – coenzyme A is derived from pantothenic acid (B vitamin). Krebs Cycle • The Krebs cycle is also called the citric acid cycle, or the tricarboxylic acid (TCA) cycle. It is a series of biochemical reactions that occur in the matrix of mitochondria (Figure 25.6). Krebs Cycle Krebs Cycle • The large amount of chemical potential energy stored in intermediate substances derived from pyruvic acid is released step by step. • The Krebs cycle involves decarboxylations and oxidations and reductions of various organic acids. • For every two molecules of acetyl CoA that enter the Krebs cycle, 6 NADH, 6 H+, and 2 FADH2 are produced by oxidation-reduction reactions, and two molecules of ATP are generated by substrate-level phosphorylation (Figure 25.6). • The energy originally in glucose and then pyruvic acid is primarily in the reduced coenzymes NADH + H+ and FADH2. Krebs Cycle (Citric Acid Cycle) • The oxidation-reduction & decarboxylation reactions occur in matrix of mitochondria. – acetyl CoA (2C) enters at top & combines with a 4C compound – 2 decarboxylation reactions peel 2 carbons off again when CO2 is formed • Potential energy (of chemical bonds) is released step by step to reduce the coenzymes (NAD+NADH & FADFADH2) that store the energy Review: Krebs Cycle • Glucose 2 acetyl CoA molecules • each Acetyl CoA molecule that enters the Krebs cycle produces – 2 molecules of C02 – 3 molecules of NADH + H+ – one molecule of ATP – one molecule of FADH2 Electron Transport Chain • The electron transport chain involves a sequence of electron carrier molecules on the inner mitochondrial membrane, capable of a series of oxidation-reduction reactions. • As electrons are passed through the chain, there is a stepwise release of energy from the electrons for the generation of ATP. • In aerobic cellular respiration, the last electron receptor of the chain is molecular oxygen (O2). This final oxidation is irreversible. • The process involves a series of oxidation-reduction reactions in which the energy in NADH + H+ and FADH2 is liberated and transferred to ATP for storage. Electron Transport Chain • Pumping of hydrogen is linked to the movement of electrons passage along the electron transport chain. • It is called chemiosmosis (Figure 25.8.) • Note location. Chemiosmosis • H+ ions are pumped from matrix into space between inner & outer membrane • High concentration of H+ is maintained outside of inner membrane • ATP synthesis occurs as H+ diffuses through a special H+ channels in the inner membrane Electron Transport Chain Steps in Electron Transport • Carriers of electron transport chain are clustered into 3 complexes that each act as a proton pump (expelling H+) • Mobile shuttles (CoQ and Cyt c) pass electrons between complexes. • The last complex passes its electrons (2H+) to oxygen to form a water molecule (H2O) Proton Motive Force & Chemiosmosis • Buildup of H+ outside the inner membrane creates + charge – The potential energy of the electrochemical gradient is called the proton motive force. • ATP synthase enzymes within H+ channels use the proton motive force to synthesize ATP from ADP and P Summary of Aerobic Cellular Respiration • The complete oxidation of glucose can be represented as follows: • C6H12O6 + 6O2 => 36 or 38ATP + 6CO2 +6H2O • During aerobic respiration, 36 or 38 ATPs can be generated from one molecule of glucose. – Two of those ATPs come from substrate-level phosphorylation in glycolysis. – Two come from substrate-level phosphorylation in the Krebs cycle. Review • Table 25.1 summarizes the ATP yield during aerobic respiration. • Figure 25.8 summarizes the sites of the principal events of the various stages of cellular respiration. Glycogenesis & Glycogenolysis • Glycogenesis – glucose storage as glycogen – 4 steps to glycogen formation in liver or skeletal muscle – stimulated by insulin • Glycogenolysis – glucose release Glycogenesis & Glycogenolysis • Glycogenesis – glucose storage as glycogen • Glycogenolysis – glucose release – not a simple reversal of steps – Phosphorylase enzyme is activated by glucagon (pancreas) & epinephrine (adrenal gland) – Glucose-6-phosphatase enzyme is only in hepatocytes so muscle can not release glucose into the serum. Gluconeogenesis • Gluconeogenesis is the conversion of protein or fat molecules into glucose (Figure 25.12). Gluconeogenesis • Glycerol (from fats) may be converted to glyceraldehyde-3phosphate and some amino acids may be converted to pyruvic acid. Both of these compounds may enter the Krebs cycle to provide energy. • Gluconeogenesis is stimulated by cortisol, thyroid hormone, epinephrine, glucagon, and human growth hormone.