Chemistry: Matter and Change
advertisement

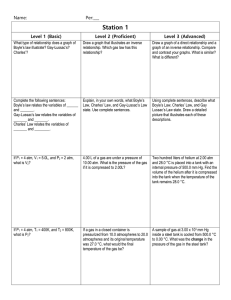
•Sections 1 & 2 Section 13.1 The Gas Laws • State the relationships among pressure, temperature, and volume of a constant amount of gas. • Apply the gas laws to problems involving the pressure, temperature, and volume of a constant amount of gas. scientific law: describes a relationship in nature that is supported by many experiments Explain the difference between a law & a theory? Section 13.1 The Gas Laws (cont.) Boyle’s law absolute zero Charles’s law Gay-Lussac’s law combined gas law For a fixed amount of gas, a change in one variable—pressure, temperature, or volume—affects the other two. What is a variable? Boyle's Law • Boyle’s law states that the volume of a fixed amount of gas held at a constant temperature varies inversely with the pressure. P1V1 = P2V2 where P = pressure and V = volume Give an everyday example of Boyle’s Law. Charles's Law • As temperature increases, so does the volume of gas when the amount of gas and pressure do not change. • Kinetic-molecular theory explains this property. Draw a series of cartoons to show what happens to a filled helium balloon as the temperature gradually increases. Charles's Law (cont.) What type of mathematical relationship is V vs T ? Charles's Law (cont.) • Absolute zero is zero on the Kelvin scale. • Charles’s law states that the volume of a given amount of gas is directly proportional to its kelvin temperature at constant pressure. Why must all temperatures be put into Kelvin units when using gas laws? Gay-Lussac's Law • Gay-Lussac’s law states that the pressure of a fixed amount of gas varies directly with the kelvin temperature when the volume remains constant. How does Gay-Lussac’s Law apply to NASCAR racing teams? Gay-Lussac's Law (cont.) What type of mathematical relationship is P vs T ? The Combined Gas Law • If all three laws (Boyle’s, Charles’, GayLussac’s) are combined then the resulting equation is the: • combined gas law states the relationship among pressure, temperature, and volume of a fixed amount of gas. In the combined gas law formula, draw a circle around Boyles’ Law, Charles’ Law & GayLussac’s Law. Shade in each circle a different color to distinguish the individual laws that make up the combined law. The Combined Gas Law (cont.) When using the combined gas law, cross out any unnecessary variable. •Ex) A child’s party inflatable has a pressure of 15.8 atm at the beginning of the party when the temperature is 33oC. Near the end of the party the temperature drops to 17oC. What is the new pressure? Solution: 1st: Convert Celsius temperatures to Kelvin units. T1 = 33 + 273 = 306 K T2 = 17 + 273 = 290. K The Combined Gas Law (cont.) 2nd: Substitute values into equation. 15.8 atm V1 306 K = P2 V2 290. K 3rd: Cross out any variables not mentioned at all. 4th: Cross multiply & solve for the unknown. (15.8atm) (290. K) = 306 K (306 K) P2 306 K P2 = 15.0 atm The Combined Gas Law (cont.)