Emission Spectrum Lab and Flame Test:
Discussion Questions
Part I:
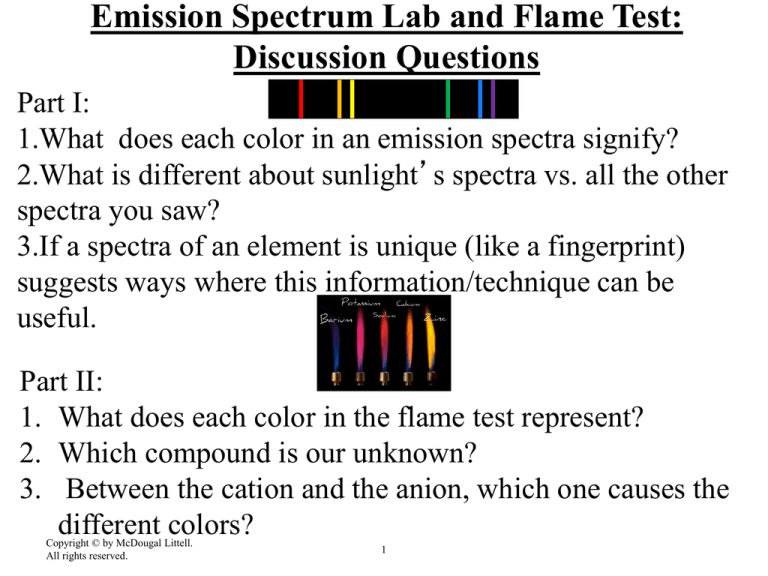
1.What does each color in an emission spectra signify?
2.What is different about sunlight’s spectra vs. all the other
spectra you saw?
3.If a spectra of an element is unique (like a fingerprint)
suggests ways where this information/technique can be
useful.
Part II:
1. What does each color in the flame test represent?
2. Which compound is our unknown?
3. Between the cation and the anion, which one causes the
different colors?
Copyright © by McDougal Littell.
All rights reserved.
1
OVERVIEW: Electromagnetic Spectrum
Copyright © by McDougal Littell.
All rights reserved.
2
1.
2.
3.
4.
5.
REVIEW: Discuss the following questions with
your partner
What are the units for wavelength, frequency
and energy?
What is the speed of light? Is it the same or
different speed for other types of
electromagnetic radiation?
What is the difference between the colors black
and white?
What is the relationship (inverse/direct)
between wavelength, frequency and energy?
Which end of the visible light spectrum has
greater frequency?
3
Photons of red and blue light (different energy
based on frequency and wavelength)
*Which wave has larger wavelength?
Higher frequency? Higher energy?
*What is relationship of wavelength/frequency/energy?
Copyright © by McDougal Littell.
All rights reserved.
4
Figure 11.12: The color of the photon emitted depends on the energy change that produces it.
Bohr’s Model of Hydrogen – electrons in
defined orbit with specific energy.
Copyright © by McDougal Littell.
All rights reserved.
5
CONSTANTS and FORMULAS
E = hn
OR E = h c/l
Where:
E=Energy (Joules)
h= Planck’s Constant 6.626 x 10-34 J·s
c= 3.0 x 108 m/s
n= frequency of light (1/s)
l = wavelength
Copyright © by McDougal
Littell. All rights reserved.
6
PRACTICE: Calculating Energy of the different wavelength
E = hn
OR E = hc/l
Where:
E=Energy (Joules)
h= Planck’s Constant 6.626 x 10-34 J-
·s
c= 3.0 x
108 m/s
n= frequency of light (1/s)
l = wavelength
Copyright © by McDougal Littell.
All rights reserved.
The blue color of fireworks
is often achieved by heating
copper (I) chloride (CuCl)
to 1200°C. The compound
emits blue light at a
wavelength (l) of
4.50 x 10-7 m.
A. What is the Energy
produced?
B. What is the frequency
of the light produced?
ANSWER: A.) 4.42 X 10-19 Joules
B.) 6.67 X 10 14 1/s
Practice Problem
An excited electron returned to its ground state and
released a photon with an energy measured to
contain 3.313 X10 -19 Joules.
i.Using the speed of light and Planck’s constant,
what is the wavelength of this light?
ii.What color is it (refer to the chart below)?
HINT:
100 nm = 1.00X10 -7 m
Copyright © by McDougal Littell.
All rights reserved.
8
Figure 11.12: The color of the photon emitted depends on the energy change that produces it.
Bohr’s Model of Hydrogen – electrons in
defined orbit with specific energy.
• Energy has a
negative value
if released
(excited to
ground)
Copyright © by McDougal Littell.
All rights reserved.
9
• Energy has a
positive value
if absorbed
(ground to
excited)
Transition of Electrons (Rydberg’s Equation)
Where:
E – Energy in Joules
R – Rydberg’s Constant of
2.178 x10 18 J
nf – final energy level
ni-- initial energy level
EXAMPLE:
An electron in a hydrogen atom falls
from the 4th energy level to the 2nd,
calculation the energy releases. What
wavelength of light is produced?
QUESTION:
Based from what we have
discussed so far, how would you
describe the structure of an
atom?
Copyright © by McDougal Littell.
All rights reserved.
12