Group 2 - Cloudfront.net
advertisement
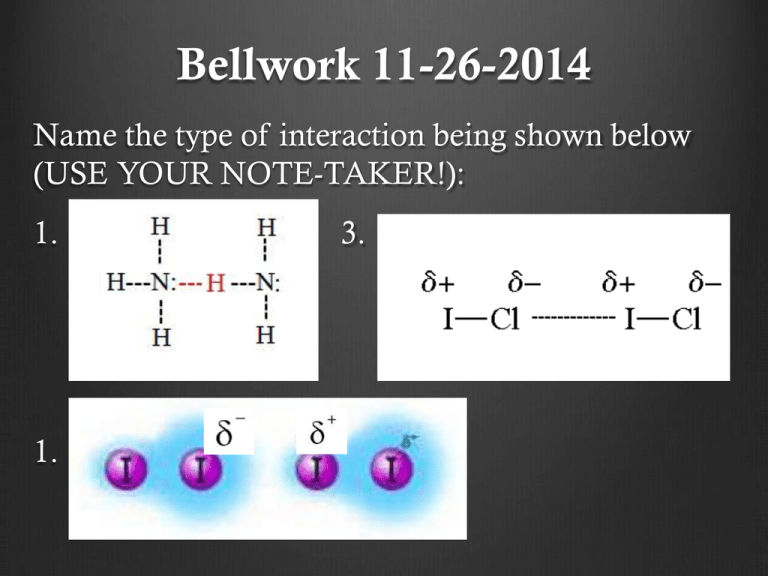
Bellwork 11-26-2014 Name the type of interaction being shown below (USE YOUR NOTE-TAKER!): 1. 3. 1. 4. Bellwork 11-26-2014 Honors Name the types of reactions below: 1. 4Fe(s) + 3O2(g) → 2Fe2O3(s) 2. Na2CO3(aq) + CaCl2(aq) → CaCO3(s) + 2NaCl(aq) 3. 2CH3OH(g) + 3O2(g) 2CO2 (g) + 4H2O(g) Announcments Labs need to be checked off today! Lab notebooks need to be in the cabinet today for labs next week! HW on intermolecular forces due next Mon/Tue Honors: HW on reactions due Mon Answer to Ionic Naming HW Monoatomic ion HW Answer to Ionic Naming HW Polyatomic ion HW 1. LiClO 2. Be(NO2)2 3. Na3AsO2 4. Ca(OH)2 5. Pb(NO3)4 6. Hg(NO3)2 7. (NH4)2SO4 8. Cr(OH)2 9. Ag(NO2)3 10. Al2(SO4)3 1. Lithium Chromate 2. Beryllium Iodate 3. Magnesium Arsenite 4. Iron (III) Phosphate 5. Aluminum Sulfite Answers to Intermolecular Forces HW Molecular Shape and Polarity Because all molecules take on different molecular shapes, some molecules may contain polar bonds and ultimately be polar OR nonpolar molecules. Draw each example with its electron pair and molecular geometry to determine if the molecule is overall polar or non-polar. Reactions Independently: answer the first question on your note-taker Share out DEMO TIME!!! Burning ethyl alcohol: C2H6O + 3O2 2CO2 + 3H2O Copper with nitric acid: Cu(s) + 4HNO3(aq) Cu(NO3)2(aq) + 2NO2(g) + 2H2O(l) Lead nitrate with potassium iodide Pb(NO3)2 (aq) + 2KI (aq) PbI2 (s) + 2KNO3 (aq) Evidence of a reaction: 1. 2. 3. 4. States: Solid (s) Liquid (l) Gas (g) Aqueous (aq) – something dissolved in water Diatomics: H2 N2 F2 O2 I2 Cl2 Br2 Bellwork 12-2-2014 Have out intermolecular forces HW 1. Write a chemical reaction for the scenario below using your knowledge of ionic compounds: Calcium reacts with sulfur to create calcium sulfide Reactant + reactant product Announcements Go over homework Intermolecular forces New groups Competition for extra credit Credit on assignment at the end of the day (everyone in group must participate and complete assignment) Groups Period 5 Group 1: Aaron, Rachel, Michael, Sarah Group 2: Claudia, Jessica, Alan Group 3: Jasmine A, Ana, Alaina Group 4: Frank, Gabby, Samantha, Ivan Group 5: Jaime, Kinshasa, Jeff, Celeste Group 6: Shirely, Cierra, Luis A., Khalil Group 7: Jasmine G, Janet, Yaquelin, Group 8: Laura,Yansi, Elba, Alex Groups Period 6 Group 1: Thania, Edward, Reino, Alex R. Group 2: Daniella, Steven, Frank, Melani Group 3: Olga, Christian, Jasmin, Jesse Group 4: Victor, Cherie, James, Nataly Groups Period 4 Group 1: Brenda, Jennifer, Silvino, Leo Group 2: Jose A., Nathalie, David, Yulisa Group 3: Yeni, April, Natalie G., Emilio Group 4: Gabby, Alejandra, Davion, Deija Group 5: Raquel, Jasson, Xochil, Joshua Group 6: Aimee, Jonathan, Jeanette Malachi, Joana Group 7: Andrew, Genesis, Edgar, Jaqueline Group 8: Sonya, Azzel, Rene, Jose. F, Groups – Period 2 Group 1: Armida, Lennesa, Daniel, Ricardo Group 2: Cristal, Joefran, Zulma Ricky Group 3: Andy, Adriana, Henry, Heneyda Group 4: Margarita, Ashley, Ricardo Heriberto Group 5: Efrain, Sandra, Alex Group 6: Miguel, Ruth, Cristo Group 7: Pedro, Ariel, Alondra Siul Group 8: Airam, Adam, Gregory Reaction Sets Set A 4Fe(s) + 3O2(g) → 2Fe2O3(s) N2(g) + 3H2(g) → 2NH3(g) 2SO2(g) + O2(g) → 2SO3(g) MgO(s)+ H2O(l) → Mg(OH)2(aq) P2O5(g) + 3H2O(l) → 2H3PO4(aq) SO3(g) + H2O(l) → H2SO4(aq) Set B MgCO3(s) → MgO(s) + CO2(g) 8Li2S(s) → 16Li(s) + S8(s) 2H2O(l) → 2H2(g) + O2(g) 2KClO3(s) → 2KCl(s) + 3O2(g) 2Na2O2(s) → 2Na2O(s) + O2(g) (NH4)2CO3(s) → 2NH3(g) + H2O(l) + CO2(g) Set C 2FeCl3(aq) + 3Zn(s) → 2Fe(s) + 3ZnCl2(aq) Mg(s) + CuSO4(aq) → MgSO4(aq) + Cu(s) 2Al(s) + 6HCl(aq) → 2AlCl (aq) + 3H (g) Cl2(g) + 2NaBr(aq) → 2NaCl(aq) + Br2(l) ZnBr2(aq) + F2(g) → ZnF2(aq) + Br2(l) 2Al(NO3)3(aq) + 3Ca(s) → 3Ca(NO3)2(aq) + 2Al(s) Set D AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq) 2HNO3(aq) + Mg(OH)2(aq) → Mg(NO3)2(aq) + 2H2O(l) Na2CO3(aq) + CaCl2(aq) → CaCO3(s) + 2NaCl(aq) FeS(s) + 2HCl(aq) → H2S(g) + FeCl2(aq) HCl(aq) + NaOH(aq) → H2O(l) + NaCl(aq) FeBr3(aq) + K3PO4(aq) → FePO4(s) + 3KBr(aq) Set E CH4(g) + 2O2(g) → CO2(g) + 2H2O(g) C10H8(g) + 12O2(g) → 10CO2(g) + 4H2O(g) 2C2H6(g) + 7O2(g) → 4CO2(g) + 6H2O(g) 2CH3OH(g) + 3O2(g) 2CO2 (g) + 4H2O(g) Definitions Synthesis – two or more simple substances combine to form a more complex one. Ex. A + B AB Decomposition – a complex substance is broken down into two or more simple substances. Ex. AB A + B Definitions Single Replacement – an element or ion moves out of one compound and into another or by itself Ex. AB + C AC + B Double Replacement – The exchange of bonds between two reacting substances. Ex. AB + CD AC + BD Definitions Combustion – burning reaction between fuel and oxygen resulting in the release of CO2 and H2O. Ex. CH4 + 2O2 CO2 + 2H2O Answers to Practice Problems Synthesis: 1. Na + Cl2 NaCl 2. K2O + H2O KOH 3. Mg + O2 MgO 4. K + P K3P Answers to Practice Problems Decomposition: 1. H2O H2 + O2 2. NaCl Na + Cl2 3. BaO Ba + O2 4. Be3N2 Be + N2 Answers to Practice Problems Single Replacement: 1. P + NaCl NaP + Cl2 2. AlSO4 + Ba Ba3(SO4)2 Al 3. Mg + CuNO3 Mg(NO3)2 + Cu 4. Na + Ca2P3 Na3P + Ca 5. NaCl + Ag AgCl + Na 6. Li + Ca3(PO4)2 Li3PO4 + Ca Answers to Practice Problems Double Replacement: 1. Cu(NO3)2 + NaCl NaNO3 + CuCl2 2. ZnI2 + AlSO4 ZnSO4 + AlI3 3. K2O + MgBr2 KBr + MgO Answers to Practice Problems Identifications: a) Synthesis b) Single replacement c) Combustion d) Decomposition e) Single replacement f) Double replacement Homework Reaction worksheet Due Th/Fri Per 2+ 4 – due Wed Lab notebooks required on Wednesday Bellwork 12-1-2014 Honors Have out HW – intermolecular forces and rxn worksheet 1. Describe how you think intermolecular forces play a role in chemical reactions. Announcements New groups Go over homework Reaction worksheet Intermolecular forces worksheet GET LAB NOTEBOOKS OUT Answers to Homework Synthesis: Mg + Cl2 MgCl2 KCl + O2 KClO3 CaO + CO2 CaCO3 Decomposition: MgCl2 Mg + Cl2 FeS Fe + S BaClO3 BaCl2 + O2 Double Replacement: AgNO3 + NaCl AgCl + NaNO3 NH4Cl + NaOH NH4OH + NaCl Pb(NO3)2 K2S PbS + KNO3 Identification: 1. Synthesis 2. Combustion 3. Double replacement 4. Decomposition Single Replacement: 5. Single Replacement Cu + AgNO3 Ag + CuNO3 6. Double replacement Ca + H2O Ca(OH)2 + H2 7. Combustion Fe + Cu(NO3)2 Fe(NO3)2 + Cu 8. Single replacement NaI + Br2 NaBr + I2 9. Synthesis 10. Decomposition Groups Period 3 Group 1: Kathy, Abner, Michael, Mariajose Group 2: Angie, Rene, Josue, Amber Group 3: Arlene, Oscar, Aleida, Rosa Group 4: Cynthia, LuisAngel, Daniel, Genesis Group 5: Fatima, Cristina, Christian, Arlen Group 6: Sheelly, Luis H., Esmeralda R., Juan Group 7: Jessica, Delia, Bryant, Yasmin Group 8: Leslie G, Jocelyne, Esmeralda A Jason, Leslie P. Honors: Mini Labs First Lab (2 pages; front back) • Title: Sugar Snake • Question: • Procedures (summarized description of the lab process 2-3 sentences): • Results: observations • Conclusion Questions: Honors: Mini Labs Second Lab (2 pages; front back) • Title: Elephant Toothpaste • Question: • Procedures (summarized description of the lab process – 2-3 sentences): • Results: observations • Conclusion Questions: As you finish… If your groups COMPLETELY finishes the first lab, I will check off all books and randomly grade ONE for the whole group WORK TOGETHER Work on the second lab and I will do the same