Flame Test Lab

Flame Test Lab
Objective: The objective of this lab is to observe different elements when they are put into a flame and to use these observations to identify unknown elements.
Background: Each element has a set of electrons that exist in energy levels (shells) around the nucleus.
This is called the element’s ground state. When substances are heated in a flame, the atoms absorb energy from the flame. This absorbed energy can cause the atom’s electrons to jump out of their shell and move into outer shells or excited energy levels. Once in these excited energy levels, electrons will return to their ground state
(original energy shells) and when they do they give off the energy that was absorbed. This energy is given off in the form of a photon (light).
The wavelength of the photon will determine the color that is emitted from the element. Because each element has a different spacing of energy levels and electrons, the possible electron transitions are
View Animation by clicking here . After viewing the animation, draw the 3 steps with labels below. unique for each element. As a result, the colors observed when a substance is heated in a flame can be used as a means of identification.
Lab Activity: You will be taking half soaked splints from the soaking container and then putting them into the flames from your Bunsen burner and observing the colors that are emitted. You will work with 8 known solutions and then 2 unknown solutions. You will use your data from the known solutions to identify the 2 unknown solutions.
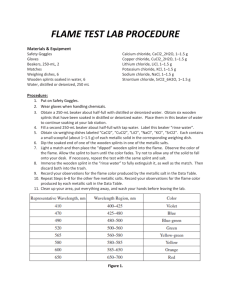
1. Put on safety goggles. These must be worn during the entire lab as the lab will require flames and potentially toxic chemicals. If there is a spill of chemicals make sure to get your teacher! If the chemicals get on your skin immediately wash with soap and water.
2. Make sure at each station you have a 250 ml beaker half filled with water to extinguish your splints and matches into. NOTHING SHOULD GO IN THE SINK TODAY! Also, make sure you have matches and splints for lighting your Bunsen burner.
3. Take a long piece of plastic wrap and tape it down at your station. This will be used to organize all of your splints. Once taped down, use a sharpie to label in a long row at the top the eight different elements and 2 unknowns. You can use the symbols which are as follows:
Ba, Ca, Cu, Pb, Li, K, Na, Sr, Unknown 1, Unknown 2
4. Take the empty beaker to the area where the solutions are located. Pick one of the element solutions to start with. Take one splint carefully out of the solution and place it into your empty beaker to carry back to your station. Place it in the appropriate spot on the plastic wrap. Make sure you know which element solution you have on your splint so that you can record the data. ONLY TAKE ONE SPLINT AT A TIME
AND THEN RETURN TO GET ANOTHER ONE. If there is solution left in your beaker, wipe it out with a paper towel before you put another splint into it. Be careful and take your time.
4. Once back at your station and with all of your splints laid out, light your Bunsen burner. Remember: light the match and then the splint before turning on the gas! The match and splint should go into waste beaker.
5. Place the wet section of each splint into the flame and observe the color that is emitted. Keep the splint in the flame until the color is not longer coming through. Once done with the splint, place it into the waste beaker. Record your data on the data table.
6. Repeat step 5 for all of the remaining splints.
7. Once you are done with all 10 splints, turn off your gas and clean up your station. Everything should be cleaned EXCEPT the waste beaker—I will take care of this later. Once cleaned, you can work with your partner to identify the unknowns.
8. Return to your seats to complete the post lab on your own!
Pre Lab:
1. Where do electrons exist in an atom?
2. How many electrons does Sodium (Na) have? Draw its electron configuration below.
3. Before an element is heated, the element exists in its normal state which is called its:
4. Define the excited state.
5. When the electrons return to their ground state after being heated, energy is emitted in what form?
6. Why does each element emit a unique color?
7. What precautions must be taken in the lab?
8. What is the purpose of the plastic wrap in the lab activity? What should be labeled on it?
9. How do you light a Bunsen burner?
10. Where should all of the lit splints go once you are done with them?
Data Table
Element Color Emitted Observations/Comments
Barium
(Ba)
Calcium
(Ca)
Copper
(Cu)
Lead
(Pb)
Lithium
(Li)
Potassium
(K)
Sodium
(Na)
Strontium
(Sr)
Unknown 1 ID?
Unknown 2 ID?
Post Lab:
1.
Which elements gave off the most striking colors?
2.
Go to ptable.com on your ipad. Find the 8 elements that were used in the lab on the periodic table.
Are these elements metals, non-metals, metalloids or a combination? How do you know this?
3.
According to your answer above, do these 8 elements typically like to give away electrons or take electrons (think back to your metals/nonmetals lab).
4.
Why do you think these 8 elements give off such vivid colors? Do you think non-metals would act the same way? Explain your answer.
5.
Can you think of a way(s) the findings of this lab can be applied in the world? Think for a minute and list a couple of possible answers:
6. Watch one or two minutes of the following video: http://www.youtube.com/watch?v=ixyyb-Ed-YA
Then pick a 10 second stretch to use to fill out the chart below.
Times of Video Order of Colors in Fireworks Elements involved in Fireworks
6.
Refer to the following article to answer the questions: http://scifun.chem.wisc.edu/chemweek/fireworks/fireworks.htm
What are the 3 forms of energy created in a firework?
7.
What causes the colors in fireworks? How does this relate to the lab that we just did?
8.
Write the order of colors of the visible spectrum from longest to shortest wavelength.
9.
Which compounds emit higher energy photons copper compounds or lithium compounds? Explain your answer.
10. What is a “star” package in a firework?
11. Fireworks obviously do not have a Bunsen burner to give them energy. What causes the fireworks to ignite?
12. If we wanted to create a red firework with silver sparkles, what elements would we need to have in our firework?
13. Describe a cool firework that you would create. Include colors and elements in the description (Extra
Credit if you draw it too in color!)